Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation
- PMID: 20627999
- PMCID: PMC2957776
- DOI: 10.1124/jpet.110.170225
Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation
Abstract
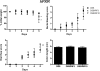
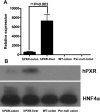
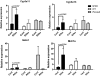
Human pregnane X receptor (PXR) has been implicated in the pathogenesis of inflammatory bowel disease (IBD). Rifaximin, a human PXR activator, is in clinical trials for treatment of IBD and has demonstrated efficacy in Crohn's disease and active ulcerative colitis. In the current study, the protective and therapeutic role of rifaximin in IBD and its respective mechanism were investigated. PXR-humanized (hPXR), wild-type, and Pxr-null mice were treated with rifaximin in the dextran sulfate sodium (DSS)-induced and trinitrobenzene sulfonic acid (TNBS)-induced IBD models to determine the protective function of human PXR activation in IBD. The therapeutic role of rifaximin was further evaluated in DSS-treated hPXR and Pxr-null mice. Results demonstrated that preadministration of rifaximin ameliorated the clinical hallmarks of colitis in DSS- and TNBS-treated hPXR mice as determined by body weight loss and assessment of diarrhea, rectal bleeding, colon length, and histology. In addition, higher survival rates and recovery from colitis symptoms were observed in hPXR mice, but not in Pxr-null mice, when rifaximin was administered after the onset of symptoms. Nuclear factor κB (NF-κB) target genes were markedly down-regulated in hPXR mice by rifaximin treatment. In vitro NF-κB reporter assays demonstrated inhibition of NF-κB activity after rifaximin treatment in colon-derived cell lines expressing hPXR. These findings demonstrated the preventive and therapeutic role of rifaximin on IBD through human PXR-mediated inhibition of the NF-κB signaling cascade, thus suggesting that human PXR may be an effective target for the treatment of IBD.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

