TGFBR2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections
- PMID: 20628007
- PMCID: PMC2972687
- DOI: 10.1093/cvr/cvq230
TGFBR2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections
Abstract
Aims: Transforming growth factor-β (TGF-β) signaling is critical for the differentiation of smooth muscle cells (SMCs) into quiescent cells expressing a full repertoire of contractile proteins. Heterozygous mutations in TGF-β receptor type II (TGFBR2) disrupt TGF-β signaling and lead to genetic conditions that predispose to thoracic aortic aneurysms and dissections (TAADs). The aim of this study is to determine the molecular mechanism by which TGFBR2 mutations cause TAADs.
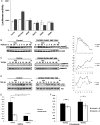
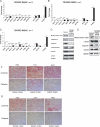
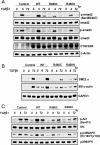
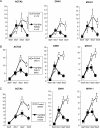
Methods and results: Using aortic SMCs explanted from patients with TGFBR2 mutations, we show decreased expression of SMC contractile proteins compared with controls. Exposure to TGF-β1 fails to increase expression of contractile genes in mutant SMCs, whereas control cells further increase expression of these genes. Analysis of fixed and frozen aortas from patients with TGFBR2 mutations confirms decreased in vivo expression of contractile proteins relative to unaffected aortas. Fibroblasts explanted from patients with TGFBR2 mutations fail to transform into mature myofibroblasts with TGF-β1 stimulation as assessed by expression of contractile proteins.
Conclusions: These data support the conclusion that heterozygous TGFBR2 mutations lead to decreased expression of SMC contractile protein in both SMCs and myofibroblasts. The failure of TGFBR2-mutant SMCs to fully express SMC contractile proteins predicts defective contractile function in these cells and aligns with a hypothesis that defective SMC contractile function contributes to the pathogenesis of TAAD.
Figures

References
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi:10.1152/physrev.00041.2003. - DOI - PubMed
-
- Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol. 2004;287:C1560–C1568. doi:10.1152/ajpcell.00221.2004. - DOI - PubMed
-
- Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet. 2004;36:855–860. doi:10.1038/ng1392. - DOI - PMC - PubMed
-
- Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, et al. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112:513–520. doi:10.1161/CIRCULATIONAHA.105.537340. - DOI - PubMed
-
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi:10.1038/ng1511. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

