Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen
- PMID: 20628030
- PMCID: PMC2907178
- DOI: 10.1158/1078-0432.CCR-10-0192
Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen
Abstract
Purpose: Most patients diagnosed with ovarian cancer will ultimately die from their disease. For this reason, novel approaches to the treatment of this malignancy are needed. Adoptive transfer of a patient's own T cells, genetically modified ex vivo through the introduction of a gene encoding a chimeric antigen receptor (CAR) targeted to a tumor-associated antigen, is a novel approach to the treatment of ovarian cancer.
Experimental design: We have generated several CARs targeted to the retained extracellular domain of MUC16, termed MUC-CD, an antigen expressed on most ovarian carcinomas. We investigate the in vitro biology of human T cells retrovirally transduced to express these CARs by coculture assays on artificial antigen-presenting cells as well as by cytotoxicity and cytokine release assays using the human MUC-CD(+) ovarian tumor cell lines and primary patient tumor cells. Further, we assess the in vivo antitumor efficacy of MUC-CD-targeted T cells in SCID-Beige mice bearing peritoneal human MUC-CD(+) tumor cell lines.
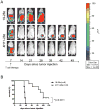
Results: CAR-modified, MUC-CD-targeted T cells exhibited efficient MUC-CD-specific cytolytic activity against both human ovarian cell and primary ovarian carcinoma cells in vitro. Furthermore, expanded MUC-CD-targeted T cells infused through either i.p. injection or i.v. infusion into SCID-Beige mice bearing orthotopic human MUC-CD(+) ovarian carcinoma tumors either delayed progression or fully eradicated disease.
Conclusion: These promising preclinical studies justify further investigation of MUC-CD-targeted T cells as a potential therapeutic approach for patients with high-risk MUC16(+) ovarian carcinomas.
Copyright 2010 AACR.
Figures


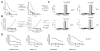

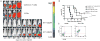
References
-
- Sun CC, Ramirez PT, Bodurka DC. Quality of life for patients with epithelial ovarian cancer. Nat Clin Pract Oncol. 2007;4(1):18–29. - PubMed
-
- Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–86. - PubMed
-
- Hwu P, Yang JC, Cowherd R, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Res. 1995;55(15):3369–73. - PubMed
-
- Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

