Duration of expression and activity of Sleeping Beauty transposase in mouse liver following hydrodynamic DNA delivery
- PMID: 20628359
- PMCID: PMC2951564
- DOI: 10.1038/mt.2010.152
Duration of expression and activity of Sleeping Beauty transposase in mouse liver following hydrodynamic DNA delivery
Abstract
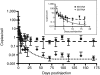
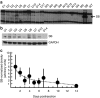
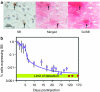
The Sleeping Beauty (SB) transposon system can direct integration of DNA sequences into mammalian genomes. The SB system comprises a transposon and transposase that "cuts" the transposon from a plasmid and "pastes" it into a recipient genome. The transposase gene may integrate very rarely and randomly into genomes, which has led to concerns that continued expression might support continued remobilization of transposons and genomic instability. Consequently, we measured the duration of SB11 transposase expression needed for remobilization to determine whether continued expression might be a problem. The SB11 gene was expressed from the plasmid pT2/mCAGGS-Luc//UbC-SB11 that contained a luciferase expression cassette in a hyperactive SB transposon. Mice were imaged and killed at periodic intervals out to 24 weeks. Over the first 2 weeks, the number of plasmids with SB11 genes and SB11 mRNA dropped about 90 and 99.9%, respectively. Expression of the luciferase reporter gene in the transposon declined about 99% and stabilized for 5 months at nearly 1,000-fold above background. In stark contrast, transposition-supporting levels of SB11 mRNA lasted only about 4 days postinfusion. Thus, within the limits of current technology, we show that SB transposons appear to be as stably integrated as their viral counterparts.
Figures



References
-
- Aiuti A., and, Roncarolo MG. Ten years of gene therapy for primary immune deficiencies. Hematology Am Soc Hematol Educ Program. 2009. pp. 682–689. - PubMed
-
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. - PubMed
-
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–458. - PubMed
-
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. - PubMed
-
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

