Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan
- PMID: 20628385
- PMCID: PMC2939781
- DOI: 10.1038/sj.bjc.6605779
Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan
Abstract
Background: A British randomised study of gemcitabine plus cisplatin (GC) combination showed promising results in biliary tract cancer (BTC) patients. In our study, we evaluated the efficacy and safety of this combination compared with gemcitabine alone (G) in Japanese BTC patients.
Methods: Overall, 84 advanced BTC patients were randomised to either cisplatin 25 mg m(-2) plus gemcitabine 1000 mg m(-2) on days 1, 8 of a 21-day cycle (GC-arm), or single-agent gemcitabine 1000 mg m(-2) on days 1, 8 and 15 of a 28-day cycle (G-arm). Treatments were repeated for at least 12 weeks until disease progression or unacceptable toxicity occurred, up to a maximum of 48 weeks.
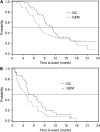
Results: A total of 83 patients were included in the analysis. For the GC and G-arms, respectively, the 1-year survival rate was 39.0 vs 31.0%, median survival time 11.2 vs 7.7 months, median progression-free survival time 5.8 vs 3.7 months and overall response rate 19.5 vs 11.9%. The most common grade 3 or 4 toxicities (GC-arm/G-arm) were neutropenia (56.1%/38.1%), thrombocytopenia (39.0%/7.1%), leukopenia (29.3%/19.0%), haemoglobin decrease (36.6%/16.7%) and gamma-GTP increase (29.3%/35.7%).
Conclusions: Gemcitabine plus cisplatin combination therapy was found to be effective and well tolerated, suggesting that it could also be a standard regimen for Japanese patients.
Conflict of interest statement
TO, KN, NM, SO, SK and JF have received honoraria, and YN, MK, JF and SN are employed by Eli Lilly Japan.
Figures

References
-
- Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ (1996) Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 2: 521–530 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

