Heat shock factors: integrators of cell stress, development and lifespan
- PMID: 20628411
- PMCID: PMC3402356
- DOI: 10.1038/nrm2938
Heat shock factors: integrators of cell stress, development and lifespan
Abstract
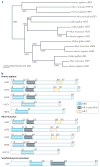
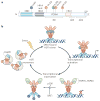
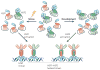
Heat shock factors (HSFs) are essential for all organisms to survive exposures to acute stress. They are best known as inducible transcriptional regulators of genes encoding molecular chaperones and other stress proteins. Four members of the HSF family are also important for normal development and lifespan-enhancing pathways, and the repertoire of HSF targets has thus expanded well beyond the heat shock genes. These unexpected observations have uncovered complex layers of post-translational regulation of HSFs that integrate the metabolic state of the cell with stress biology, and in doing so control fundamental aspects of the health of the proteome and ageing.
Conflict of interest statement
The authors declare competing financial interests: see web version for details.
Figures

References
-
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experimentia. 1962;18:571–573.
-
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. - PubMed
-
- Pelham HRB. A regulatory upstream promoter element in the Drosophila hsp70 heat-shock gene. Cell. 1982;30:517–528. - PubMed
-
- Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984;311:81–84. - PubMed
-
- Parker CS, Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp70 gene. Cell. 1984;37:273–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

