Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures
- PMID: 20628567
- PMCID: PMC2900298
- DOI: 10.1371/journal.ppat.1000991
Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures
Abstract
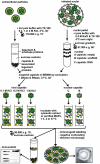
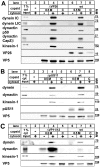
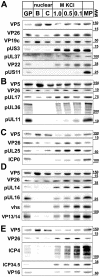
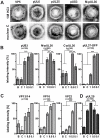
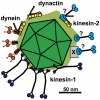
Many viruses depend on host microtubule motors to reach their destined intracellular location. Viral particles of neurotropic alphaherpesviruses such as herpes simplex virus 1 (HSV1) show bidirectional transport towards the cell center as well as the periphery, indicating that they utilize microtubule motors of opposing directionality. To understand the mechanisms of specific motor recruitment, it is necessary to characterize the molecular composition of such motile viral structures. We have generated HSV1 capsids with different surface features without impairing their overall architecture, and show that in a mammalian cell-free system the microtubule motors dynein and kinesin-1 and the dynein cofactor dynactin could interact directly with capsids independent of other host factors. The capsid composition and surface was analyzed with respect to 23 structural proteins that are potentially exposed to the cytosol during virus assembly or cell entry. Many of these proteins belong to the tegument, the hallmark of all herpesviruses located between the capsid and the viral envelope. Using immunoblots, quantitative mass spectrometry and quantitative immunoelectron microscopy, we show that capsids exposing inner tegument proteins such as pUS3, pUL36, pUL37, ICP0, pUL14, pUL16, and pUL21 recruited dynein, dynactin, kinesin-1 and kinesin-2. In contrast, neither untegumented capsids exposing VP5, VP26, pUL17 and pUL25 nor capsids covered by outer tegument proteins such as vhs, pUL11, ICP4, ICP34.5, VP11/12, VP13/14, VP16, VP22 or pUS11 bound microtubule motors. Our data suggest that HSV1 uses different structural features of the inner tegument to recruit dynein or kinesin-1. Individual capsids simultaneously accommodated motors of opposing directionality as well as several copies of the same motor. Thus, these associated motors either engage in a tug-of-war or their activities are coordinately regulated to achieve net transport either to the nucleus during cell entry or to cytoplasmic membranes for envelopment during assembly.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Sodeik B. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 2000;8:465–472. - PubMed
-
- Smith GA, Enquist LW. BREAK INS AND BREAK OUTS: Viral Interactions with the Cytoskeleton of Mammalian Cells. Annu Rev Cell Dev Biol. 2002;18:135–161. - PubMed
-
- Greber UF, Way M. A Superhighway to Virus Infection. Cell. 2006;124:741–756. - PubMed
-
- Radtke K, Döhner K, Sodeik B. Viral interactions with the cytoskeleton: A hitchhiker's guide to the cell. Cellular Microbiology. 2006;8:387–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

