Inositol hexakisphosphate-induced autoprocessing of large bacterial protein toxins
- PMID: 20628577
- PMCID: PMC2900308
- DOI: 10.1371/journal.ppat.1000942
Inositol hexakisphosphate-induced autoprocessing of large bacterial protein toxins
Abstract
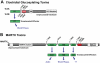
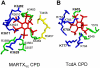
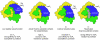
Large bacterial protein toxins autotranslocate functional effector domains to the eukaryotic cell cytosol, resulting in alterations to cellular functions that ultimately benefit the infecting pathogen. Among these toxins, the clostridial glucosylating toxins (CGTs) produced by Gram-positive bacteria and the multifunctional-autoprocessing RTX (MARTX) toxins of Gram-negative bacteria have distinct mechanisms for effector translocation, but a shared mechanism of post-translocation autoprocessing that releases these functional domains from the large holotoxins. These toxins carry an embedded cysteine protease domain (CPD) that is activated for autoprocessing by binding inositol hexakisphosphate (InsP(6)), a molecule found exclusively in eukaryotic cells. Thus, InsP(6)-induced autoprocessing represents a unique mechanism for toxin effector delivery specifically within the target cell. This review summarizes recent studies of the structural and molecular events for activation of autoprocessing for both CGT and MARTX toxins, demonstrating both similar and potentially distinct aspects of autoprocessing among the toxins that utilize this method of activation and effector delivery.
Conflict of interest statement
KJFS has a pending patent application that describes use of the CPD for biotechnological applications.
Figures
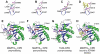
References
-
- Wooldridge K, editor. Norfolk, UK: Caister Academic Press; 2009. Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis.511
-
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

