Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice
- PMID: 20628601
- PMCID: PMC2900205
- DOI: 10.1371/journal.pone.0011484
Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice
Abstract
Background: Infections may be associated with exacerbation of allergic and autoimmune diseases. Paradoxically, epidemiological and experimental data have shown that some microorganisms can also prevent these pathologies. This observation is at the origin of the hygiene hypothesis according to which the decline of infections in western countries is at the origin of the increased incidence of both Th1-mediated autoimmune diseases and Th2-mediated allergic diseases over the last decades. We have tested whether Toll-like receptor (TLR) stimulation can recapitulate the protective effect of infectious agents on allergy and autoimmunity.
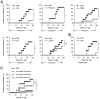
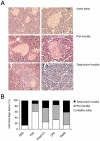
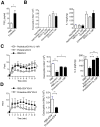
Methods and findings: Here, we performed a systematic study of the disease-modifying effects of a set of natural or synthetic TLR agonists using two experimental models, ovalbumin (OVA)-induced asthma and spontaneous autoimmune diabetes, presenting the same genetic background of the non obese diabetic mouse (NOD) that is highly susceptible to both pathologies. In the same models, we also investigated the effect of probiotics. Additionally, we examined the effect of the genetic invalidation of MyD88 on the development of allergic asthma and spontaneous diabetes. We demonstrate that multiple TLR agonists prevent from both allergy and autoimmunity when administered parenterally. Probiotics which stimulate TLRs also protect from these two diseases. The physiological relevance of these findings is further suggested by the major acceleration of OVA-induced asthma in MyD88 invalidated mice. Our results strongly indicate that the TLR-mediated effects involve immunoregulatory cytokines such as interleukin (IL)-10 and transforming growth factor (TGF)-beta and different subsets of regulatory T cells, notably CD4+CD25+FoxP3+ T cells for TLR4 agonists and NKT cells for TLR3 agonists.
Conclusions/significance: These observations demonstrate that systemic administration of TLR ligands can suppress both allergic and autoimmune responses. They provide a plausible explanation for the hygiene hypothesis. They also open new therapeutic perspectives for the prevention of these pathologies.
Conflict of interest statement
Figures

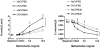




References
-
- Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. - PubMed
-
- Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. - PubMed
-
- Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. Faseb J. 2004;18:534–536. - PubMed
-
- Lang KS, Recher M, Junt T, Navarini AA, Harris NL, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

