The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane
- PMID: 20628612
- PMCID: PMC2900216
- DOI: 10.1371/journal.pone.0011489
The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane
Abstract
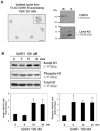
The mammalian type I gonadotropin releasing hormone receptor (GnRH-R) is a structurally unique G protein-coupled receptor (GPCR) that lacks cytoplasmic tail sequences and displays inefficient plasma membrane expression (PME). Compared to its murine counterparts, the primate type I receptor is inefficiently folded and retained in the endoplasmic reticulum (ER) leading to a further reduction in PME. The decrease in PME and concomitant increase in intracellular localization of the mammalian GnRH-RI led us to characterize the spatial distribution of the human and mouse GnRH receptors in two human cell lines, HEK 293 and HTR-8/SVneo. In both human cell lines we found the receptors were expressed in the cytoplasm and were associated with the ER and nuclear membrane. A molecular analysis of the receptor protein sequence led us to identify a putative monopartite nuclear localization sequence (NLS) in the first intracellular loop of GnRH-RI. Surprisingly, however, neither the deletion of the NLS nor the addition of the Xenopus GnRH-R cytoplasmic tail sequences to the human receptor altered its spatial distribution. Finally, we demonstrate that GnRH treatment of nuclei isolated from HEK 293 cells expressing exogenous GnRH-RI triggers a significant increase in the acetylation and phosphorylation of histone H3, thereby revealing that the nuclear-localized receptor is functional. Based on our findings, we conclude that the mammalian GnRH-RI is an intracellular GPCR that is expressed on the nuclear membrane. This major and novel discovery causes us to reassess the signaling potential of this physiologically and clinically important receptor.
Conflict of interest statement
Figures


Similar articles
-
Combined modification of intracellular and extracellular loci on human gonadotropin-releasing hormone receptor provides a mechanism for enhanced expression.Endocrine. 2000 Dec;13(3):401-7. doi: 10.1385/ENDO:13:3:401. Endocrine. 2000. PMID: 11216654
-
Desensitization and internalization of human and xenopus gonadotropin-releasing hormone receptors expressed in alphaT4 pituitary cells using recombinant adenovirus.Endocrinology. 2000 Dec;141(12):4564-75. doi: 10.1210/endo.141.12.7813. Endocrinology. 2000. PMID: 11108269
-
Gonadotropin-releasing hormone receptors with intracellular carboxyl-terminal tails undergo acute desensitization of total inositol phosphate production and exhibit accelerated internalization kinetics.J Biol Chem. 1998 May 8;273(19):11472-7. doi: 10.1074/jbc.273.19.11472. J Biol Chem. 1998. PMID: 9565559
-
Signalling, cycling and desensitisation of gonadotrophin-releasing hormone receptors.J Endocrinol. 2002 Apr;173(1):1-11. doi: 10.1677/joe.0.1730001. J Endocrinol. 2002. PMID: 11927379 Review.
-
The gonadotrophin-releasing hormone receptor: signalling, cycling and desensitisation.Arch Physiol Biochem. 2002 Apr;110(1-2):113-22. doi: 10.1076/apab.110.1.113.893. Arch Physiol Biochem. 2002. PMID: 11935408 Review.
Cited by
-
Location Bias as Emerging Paradigm in GPCR Biology and Drug Discovery.iScience. 2020 Oct 7;23(10):101643. doi: 10.1016/j.isci.2020.101643. eCollection 2020 Oct 23. iScience. 2020. PMID: 33103080 Free PMC article. Review.
-
Association of miR-938G>A Polymorphisms with Primary Ovarian Insufficiency (POI)-Related Gene Expression.Int J Mol Sci. 2017 Jun 12;18(6):1255. doi: 10.3390/ijms18061255. Int J Mol Sci. 2017. PMID: 28604625 Free PMC article.
-
Using automated imaging to interrogate gonadotrophin-releasing hormone receptor trafficking and function.Mol Cell Endocrinol. 2011 Jan 15;331(2):194-204. doi: 10.1016/j.mce.2010.07.008. Epub 2010 Aug 3. Mol Cell Endocrinol. 2011. PMID: 20688134 Free PMC article. Review.
-
Nuclear localization drives α1-adrenergic receptor oligomerization and signaling in cardiac myocytes.Cell Signal. 2012 Mar;24(3):794-802. doi: 10.1016/j.cellsig.2011.11.014. Epub 2011 Nov 18. Cell Signal. 2012. PMID: 22120526 Free PMC article.
-
G protein-coupled receptors in the hypothalamic paraventricular and supraoptic nuclei--serpentine gateways to neuroendocrine homeostasis.Front Neuroendocrinol. 2012 Jan;33(1):45-66. doi: 10.1016/j.yfrne.2011.07.002. Epub 2011 Jul 23. Front Neuroendocrinol. 2012. PMID: 21802439 Free PMC article. Review.
References
-
- Cheng KW, Nathwani PS, Leung PC. Regulation of human gonadotropin-releasing hormone receptor gene expression in placental cells. Endocrinology. 2000;141:2340–2349. - PubMed
-
- Cavanagh PC, Dunk C, Pampillo M, Szereszewski JM, Taylor JE, et al. Gonadotropin-releasing hormone-regulated chemokine expression in human placentation. Am J Physiol Cell Physiol. 2009;297:C17–27. - PubMed
-
- Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, et al. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem. 2000;275:9193–9200. - PubMed
-
- Maudsley S, Davidson L, Pawson AJ, Chan R, Lopez de Maturana R, et al. Gonadotropin-releasing hormone (GnRH) antagonists promote proapoptotic signaling in peripheral reproductive tumor cells by activating a Galphai-coupling state of the type I GnRH receptor. Cancer Res. 2004;64:7533–7544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

