Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice
- PMID: 20628647
- PMCID: PMC2898813
- DOI: 10.1371/journal.pone.0011468
Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice
Abstract
Background: Aging results in a progressive loss of skeletal muscle, a condition known as sarcopenia. Mitochondrial DNA (mtDNA) mutations accumulate with aging in skeletal muscle and correlate with muscle loss, although no causal relationship has been established.
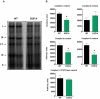
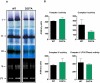
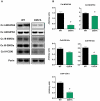
Methodology/principal findings: We investigated the relationship between mtDNA mutations and sarcopenia at the gene expression and biochemical levels using a mouse model that expresses a proofreading-deficient version (D257A) of the mitochondrial DNA Polymerase gamma, resulting in increased spontaneous mtDNA mutation rates. Gene expression profiling of D257A mice followed by Parametric Analysis of Gene Set Enrichment (PAGE) indicates that the D257A mutation is associated with a profound downregulation of gene sets associated with mitochondrial function. At the biochemical level, sarcopenia in D257A mice is associated with a marked reduction (35-50%) in the content of electron transport chain (ETC) complexes I, III and IV, all of which are partly encoded by mtDNA. D257A mice display impaired mitochondrial bioenergetics associated with compromised state-3 respiration, lower ATP content and a resulting decrease in mitochondrial membrane potential (Deltapsim). Surprisingly, mitochondrial dysfunction was not accompanied by an increase in mitochondrial reactive oxygen species (ROS) production or oxidative damage.
Conclusions/significance: These findings demonstrate that mutations in mtDNA can be causal in sarcopenia by affecting the assembly of functional ETC complexes, the lack of which provokes a decrease in oxidative phosphorylation, without an increase in oxidative stress, and ultimately, skeletal muscle apoptosis and sarcopenia.
Conflict of interest statement
Figures


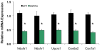



References
-
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–916. - PubMed
-
- Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S40–47. - PubMed
-
- Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. - PubMed
-
- Alexeyev MF, Ledoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci (Lond) 2004;107:355–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

