Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects
- PMID: 20628650
- PMCID: PMC2898816
- DOI: 10.1371/journal.pone.0011414
Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects
Abstract
Background: Anecdotal information and case reports suggest that intravenously administered vitamin C is used by Complementary and Alternate Medicine (CAM) practitioners. The scale of such use in the U.S. and associated side effects are unknown.
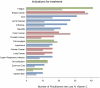
Methods and findings: We surveyed attendees at annual CAM Conferences in 2006 and 2008, and determined sales of intravenous vitamin C by major U.S. manufacturers/distributors. We also queried practitioners for side effects, compiled published cases, and analyzed FDA's Adverse Events Database. Of 199 survey respondents (out of 550), 172 practitioners administered IV vitamin C to 11,233 patients in 2006 and 8876 patients in 2008. Average dose was 28 grams every 4 days, with 22 total treatments per patient. Estimated yearly doses used (as 25 g/50 ml vials) were 318,539 in 2006 and 354,647 in 2008. Manufacturers' yearly sales were 750,000 and 855,000 vials, respectively. Common reasons for treatment included infection, cancer, and fatigue. Of 9,328 patients for whom data is available, 101 had side effects, mostly minor, including lethargy/fatigue in 59 patients, change in mental status in 21 patients and vein irritation/phlebitis in 6 patients. Publications documented serious adverse events, including 2 deaths in patients known to be at risk for IV vitamin C. Due to confounding causes, the FDA Adverse Events Database was uninformative. Total numbers of patients treated in the US with high dose vitamin C cannot be accurately estimated from this study.
Conclusions: High dose IV vitamin C is in unexpectedly wide use by CAM practitioners. Other than the known complications of IV vitamin C in those with renal impairment or glucose 6 phosphate dehydrogenase deficiency, high dose intravenous vitamin C appears to be remarkably safe. Physicians should inquire about IV vitamin C use in patients with cancer, chronic, untreatable, or intractable conditions and be observant of unexpected harm, drug interactions, or benefit.
Conflict of interest statement
Figures
Similar articles
-
Harm of IV High-Dose Vitamin C Therapy in Adult Patients: A Scoping Review.Crit Care Med. 2020 Jul;48(7):e620-e628. doi: 10.1097/CCM.0000000000004396. Crit Care Med. 2020. PMID: 32404636
-
Anaphylactoid reactions to vitamin K.J Thromb Thrombolysis. 2001 Apr;11(2):175-83. doi: 10.1023/a:1011237019082. J Thromb Thrombolysis. 2001. PMID: 11406734 Review.
-
Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany.In Vivo. 2011 Nov-Dec;25(6):983-90. In Vivo. 2011. PMID: 22021693
-
Retrospective Evaluation of Clinical Experience With Intravenous Ascorbic Acid in Patients With Cancer.Integr Cancer Ther. 2018 Sep;17(3):912-920. doi: 10.1177/1534735418775809. Epub 2018 May 17. Integr Cancer Ther. 2018. PMID: 29771164 Free PMC article.
-
Safety of vitamin C in sepsis: a neglected topic.Curr Opin Crit Care. 2019 Aug;25(4):329-333. doi: 10.1097/MCC.0000000000000622. Curr Opin Crit Care. 2019. PMID: 31107310 Review.
Cited by
-
High concentrations of L-ascorbic acid specifically inhibit the growth of human leukemic cells via downregulation of HIF-1α transcription.PLoS One. 2013 Apr 23;8(4):e62717. doi: 10.1371/journal.pone.0062717. Print 2013. PLoS One. 2013. PMID: 23626851 Free PMC article.
-
Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction.Redox Rep. 2016 Mar;21(2):75-83. doi: 10.1179/1351000215Y.0000000018. Epub 2016 Feb 24. Redox Rep. 2016. PMID: 26066587 Free PMC article. Clinical Trial.
-
Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes - role of the redox-regulated transcription factor Nrf2.BMC Complement Altern Med. 2011 Jan 3;11:1. doi: 10.1186/1472-6882-11-1. BMC Complement Altern Med. 2011. PMID: 21199573 Free PMC article.
-
Restoration of 5-hydroxymethylcytosine by ascorbate blocks kidney tumour growth.EMBO Rep. 2018 Aug;19(8):e45401. doi: 10.15252/embr.201745401. Epub 2018 Jun 28. EMBO Rep. 2018. PMID: 29959161 Free PMC article.
-
Potential Antitumor Activity of 2-O-α-d-Glucopyranosyl-6-O-(2-Pentylheptanoyl)-l-Ascorbic Acid.Int J Mol Sci. 2018 Feb 10;19(2):535. doi: 10.3390/ijms19020535. Int J Mol Sci. 2018. PMID: 29439410 Free PMC article.
References
-
- Nutrition Business Journal. NBJ's Supplement Business Report 2008: An Analysis of Markets, Trends, Competition and Strategy in the U.S. Dietary Supplement Industry. Figure 3–33(Table 1); Figure 4–18. Boulder, CO: Penton Media INC. Lifestyle Division. New Hope Natural Media; 2008.
-
- Klenner FR. The treatment of poliomyelitis and other virus diseases with vitamin C. South Med Surg. 1949;111:209–14. - PubMed
-
- Klenner FR. Massive doses of vitamin C and the virus diseases. South Med Surg. 1951;113:101–7. - PubMed
-
- Calleja HB, Brooks RH. Acute hepatitis treated with high doses of vitamin C. Report of a case. Ohio Med. 1960;56:821–23. - PubMed
-
- Klenner FR. Observations on the dose and administration of ascorbic acid when employed beyond the range of a vitamin in human pathology. Journal of Applied Nutrition. 1971;23:61–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

