Imaging brain regional and cortical laminar effects of selective D3 agonists and antagonists
- PMID: 20628733
- PMCID: PMC3822611
- DOI: 10.1007/s00213-010-1924-6
Imaging brain regional and cortical laminar effects of selective D3 agonists and antagonists
Abstract
Rationale: Dopamine D3 receptors (D3R) may be important therapeutic targets for both drug abuse and dyskinesias in Parkinson's disease; however, little is known about their functional circuitry.
Objectives: We wished to determine if D3R antagonists SB-277011 and PG-01037 and D3R-preferring agonist 7-OH-DPAT are D3R selective in vivo. We further wished to characterize the response to D3R drugs using whole brain imaging to identify novel D3R circuitry.
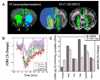
Methods: We investigated D3R circuitry in rats using pharmacologic MRI and challenge with selective D3R antagonists and agonist at various doses to examine regional changes in cerebral blood volume (CBV). We compared regional activation patterns with D2R/D3R agonists, as well as with prior studies of mRNA expression and autoradiography.
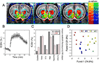
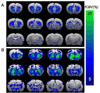
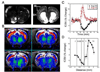
Results: D3R antagonists induced positive CBV changes and D3R agonist negative CBV changes in brain regions including nucleus accumbens, infralimbic cortex, thalamus, interpeduncular region, hypothalamus, and hippocampus (strongest in subiculum). All D3R-preferring drugs showed markedly greater responses in nucleus accumbens than in caudate/putamen consistent with D3R selectivity and contrary to what was observed with D2R agonists. At high doses of D3R agonist, functional changes were differentiated across cortical laminae, with layer V-VI yielding positive CBV changes and layer IV yielding negative CBV changes. These results are not inconsistent with differential D1R and D3R innervation in these layers respectively showed previously using post-mortem techniques.
Conclusions: MRI provides a new tool for testing the in vivo selectivity of novel D3R dopaminergic ligands where radiolabels may not be available. Further, the functional D3R circuitry strongly involves hypothalamus and subiculum as well as the limbic striatum.
Figures



References
-
- Ahlenius S, Salmi P. Behavioral and biochemical effects of the dopamine D3 receptor-selective ligand, 7-OH-DPAT, in the normal and the reserpine-treated rat. Eur J Pharmacol. 1994;260:177–181. - PubMed
-
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. - PubMed
-
- Aretha CW, Galloway MP. Dopamine autoreceptor reserve in vitro: possible role of dopamine D3 receptors. Eur J Pharmacol. 1996;305:119–122. - PubMed
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. - PubMed
-
- Baker LE, Hood CA, Heidema AM. Assessment of D3 versus D2 receptor modulation of the discriminative stimulus effects of (+)-7-OH-DPAT in rats. Behav Pharmacol. 1999;10:717–722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

