Training family physicians in shared decision making for the use of antibiotics for acute respiratory infections: a pilot clustered randomized controlled trial
- PMID: 20629764
- PMCID: PMC3073122
- DOI: 10.1111/j.1369-7625.2010.00616.x
Training family physicians in shared decision making for the use of antibiotics for acute respiratory infections: a pilot clustered randomized controlled trial
Abstract
Background: Experts estimate that the prevalence of antibiotics use exceeds the prevalence of bacterial acute respiratory infections (ARIs).
Objective: To develop, adapt and validate DECISION+ and estimate its impact on the decision of family physicians (FPs) and their patients on whether to use antibiotics for ARIs.
Design: Two-arm parallel clustered pilot randomized controlled trial.
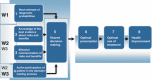
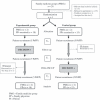
Setting and participants: Four family medicine groups were randomized to immediate DECISION+ participation (the experimental group) or delayed DECISION+ participation (the control group). Thirty-three FPs and 459 patients participated.
Intervention: DECISION+ is a multiple-component, continuing professional development program in shared decision making that addresses the use of antibiotics for ARIs.
Main outcome measures: Throughout the pilot trial, DECISION+ was adapted in response to participant feedback. After the consultation, patients and FPs independently self-reported the decision (immediate use, delayed use, or no use of antibiotics) and its quality. Agreement between their decisional conflict was assessed. Two weeks later, patients assessed their decisional regret and health status.
Results: Compared to the control group, the experimental group reduced its immediate use of antibiotics (49 vs. 33% absolute difference = 16%; P = 0.08). Decisional conflict agreement was stronger in the experimental group (absolute difference of Pearson's r = 0.26; P = 0.06). Decisional regret and perceptions of the quality of the decision and of health status in the two groups were similar.
Discussion and conclusions: DECISION+ was developed successfully and appears to reduce the use of antibiotics for ARIs without affecting patients' outcomes. A larger trial is needed to confirm this observation.
© 2010 Blackwell Publishing Ltd.
Figures
References
-
- Hing E, Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2004 summary. Advance Data, 2006; 372: 1–33. - PubMed
-
- Alberta Clinical Practice Guideline Working Group . Guideline for the Diagnosis and Treatment of Acute Pharyngitis. Alberta, Canada: Alberta Clinical Practice Guidelines Program, 1999.
-
- Nash DR, Harman J, Wald ER, Kelleher KJ. Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections. Archives of Pediatrics and Adolescent Medicine, 2002; 156: 1114–1119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous