Protective effect of single-dose adjuvanted pandemic influenza vaccine in children
- PMID: 20629771
- PMCID: PMC5964543
- DOI: 10.1111/j.1750-2659.2010.00146.x
Protective effect of single-dose adjuvanted pandemic influenza vaccine in children
Abstract
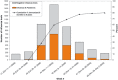
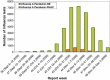
Background: During the first wave of A/California/7/2009(H1N1) influenza, high rates of hospitalization in children under 5 years were seen in many countries. Subsequent policies for vaccinating children varied in both type of vaccine and number of doses. In Canada, children 36 months to <10 years received a single dose of 0.25 ml of the GSK adjuvanted vaccine (Arepanrix) equivalent to 1.9 microg HA. Children 6 months to 35 months received two doses as did those 36-119 months with chronic medical conditions.
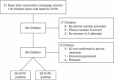
Method: We conducted a community-based case-control vaccine effectiveness (VE) review of children under 10 years with influenza like illness who were tested for H1N1 infection at the central provincial laboratory. Laboratory-confirmed influenza was the primary outcome, and vaccination status the primary exposure to assess VE after a single 0.25-ml dose.
Results: If vaccination was designated to be effective after 14 days, no vaccinated child had laboratory-confirmed influenza compared to 38% of controls. The VE of 100% was statistically significant for children <10 years of age and <5 years considered separately. If vaccination was considered effective after 10 days, VE dropped to 96% overall but was statistically significant and over 90% in all age subgroups, including those under 36 months.
Conclusions: A single 0.25-ml dose of the GSK adjuvanted vaccine (Arepanrix) protects children against laboratory-confirmed pandemic influenza potentially avoiding any increased reactogenicity associated with second doses. Adjuvanted vaccines offer hope for improved seasonal vaccines in the future.
Figures
References
-
- Baker MG, Kelly H, Wilson N Pandemic H1N1 influenza lessons from the southern hemisphere. Euro Surveill 2009;14(42): pii=19370. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19370. - PubMed
-
- New South Wales public health network . Progression and impact of the first winter wave of the 2009 pandemic H1N1 influenza in New South Wales, Australia. Euro Surveill 2009;14(42):pii=19365. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19365. - PubMed
-
- Smith S, Demicheli V, Di Pietrantonj C et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2006:CD004879 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

