Lessons from the first year of the WAIVE study investigating the protective effect of influenza vaccine against laboratory-confirmed influenza in hospitalised children aged 6-59 months
- PMID: 20629773
- PMCID: PMC5964547
- DOI: 10.1111/j.1750-2659.2010.00141.x
Lessons from the first year of the WAIVE study investigating the protective effect of influenza vaccine against laboratory-confirmed influenza in hospitalised children aged 6-59 months
Abstract
Background: Influenza is major cause of paediatric hospitalisation. Influenza vaccine was offered to all children aged 6-59 months resident in Western Australia in 2008, and we wished to evaluate the effectiveness of this immunisation programme.
Objectives: To assess the practicalities of a nested matched case-control design to estimate the protective effect of inactivated influenza vaccination in hospitalised children aged 6-59 months.
Methods: Cases were hospitalised children with laboratory-confirmed influenza, while matched controls were recruited from children admitted for an acute non-respiratory illness. We estimated influenza vaccine effectiveness (VE) against influenza as 1--the adjusted odds ratio from multivariate logistic regression.
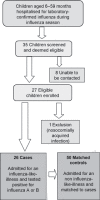
Results: The 2008 influenza season was characterised by a late peak and a predominance of influenza virus B. We recruited 26 hospitalised patients with laboratory-confirmed influenza and 50 matched controls. The proportion of cases who were fully vaccinated was 7% versus 30% of controls giving an adjusted VE of 83% (95% CI--54 to 98).
Conclusions: Recruiting sufficient controls was problematic and in the future, we will select controls hospitalised for an influenza-like-illness but influenza negative by laboratory PCR testing. The VE estimate was high but non-significant, reflecting the low number of cases.
Figures
References
-
- Brotherton J, Wang H, Schaffer A et al. Vaccine preventable diseases and vaccination coverage in Australia, 2003 to 2005. Commun Dis Intell 2007; 31(Suppl):S1–S152. - PubMed
-
- Moore H, Burgner D, Carville K, Jacoby P, Richmond P, Lehmann D. Diverging trends for lower respiratory infections in non‐Aboriginal and Aboriginal children. J Paediatr Child Health 2007; 43(6):451–457. - PubMed
-
- Smith S, Demicheli V, Di Pietrantonj C et al. Vaccines for preventing influenza in healthy children.[see comment]. Cochrane Database Syst Rev 2006; 1:CD004879. - PubMed
-
- Bridges C, Fukuda K, Uyeki T, Cox N, Singleton J. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2002; 51(RR03):1–31. - PubMed
-
- Fiore AE, Shay DK, Haber P et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56 (RR‐6):1–54. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical