Experience of safety monitoring in the context of a prospective observational study of artemether-lumefantrine in rural Tanzania: lessons learned for pharmacovigilance reporting
- PMID: 20630066
- PMCID: PMC2924868
- DOI: 10.1186/1475-2875-9-205
Experience of safety monitoring in the context of a prospective observational study of artemether-lumefantrine in rural Tanzania: lessons learned for pharmacovigilance reporting
Abstract
Objectives: To identify and implement strategies that help meet safety monitoring requirements in the context of an observational study for artemether-lumefantrine (AL) administered as first-line treatment for uncomplicated malaria in rural Tanzania.
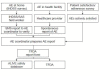
Methods: Pharmacovigilance procedures were developed through collaboration between the investigating bodies, the relevant regulatory authority and the manufacturer of AL. Training and refresher sessions on the pharmacovigilance system were provided for healthcare workers from local health facilities and field recorders of the Ifakara Health Demographic Surveillance System (IHDSS). Three distinct channels for identification of adverse events (AEs) and serious adverse events (SAEs) were identified and implemented. Passive reporting took place through IHDSS and health care facilities, starting in October 2007. The third channel was through solicited reporting that was included in the context of a survey on AL as part of the ALIVE (Artemether-Lumefantrine In Vulnerable patients: Exploring health impact) study (conducted only in March-April 2008).
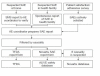
Results: Training was provided for 40 healthcare providers (with refresher training 18 months later) and for six field recorders. During the period 1st September 2007 to 31st March 2010, 67 AEs were reported including 52 under AL, five under sulphadoxine-pyrimethamine, one under metakelfin, two after antibiotics; the remaining seven were due to anti-pyretic or anti-parasite medications. Twenty patients experienced SAEs; in 16 cases, a relation to AL was suspected. Six of the 20 cases were reported within 24 hours of occurrence.
Discussion: Safety monitoring and reporting is possible even in settings with weak health infrastructure. Reporting can be enhanced by regular and appropriate training of healthcare providers. SMS text alerts provide a practical solution to communication challenges.
Conclusion: Experience gained in this setting could help to improve spontaneous reporting of AEs and SAEs to health authorities or marketing authorization holders.
Figures

References
-
- Edwards IR. Safety monitoring of new anti-malarials in immediate post-marketing phase. Med Trop (Mars) 1998;58:93–96. - PubMed
-
- WHO. The importance of pharmacovigilance. 2010. http://apps.who.int/medicinedocs/en/d/Js4893e/
-
- Cobert B, Biron P. Pharmacovigilance from A to Z. Adverse drug event surveillance. Oxford: Blackwell Science; 2002.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

