Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells
- PMID: 20630104
- PMCID: PMC2914703
- DOI: 10.1186/1471-2407-10-370
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells
Abstract
Background: Chloroquine (CQ), the worldwide used anti-malarial drug, has recently being focused as a potential anti-cancer agent as well as a chemosensitizer when used in combination with anti-cancer drugs. It has been shown to inhibit cell growth and/or to induce cell death in various types of cancer. 5-Fluorouracil (5-FU) is the chemotherapeutic agent of first choice in colorectal cancer, but in most cases, resistance to 5-FU develops through various mechanisms. Here, we focused on the combination of CQ as a mechanism to potentiate the inhibitory effect of 5-FU on human colon cancer cells.
Methods: HT-29 cells were treated with CQ and/or 5-FU, and their proliferative ability, apoptosis and autophagy induction effects, and the affection of the cell cycle were evaluated. The proliferative ability of HT-29 was analyzed by the MTS assay. Apoptosis was quantified by flow-cytometry after double-staining of the cells with AnnexinV/PI. The cell cycle was evaluated by flow-cytometry after staining of cells with PI. Autophagy was quantified by flow-cytometry and Western blot analysis. Finally, to evaluate the fate of the cells treated with CQ and/or 5-FU, the colony formation assay was performed.
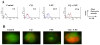
Results: 5-FU inhibited the proliferative activity of HT-29 cells, which was mostly dependent on the arrest of the cells to the G0/G1-phase but also partially on apoptosis induction, and the effect was potentiated by CQ pre-treatment. The potentiation of the inhibitory effect of 5-FU by CQ was dependent on the increase of p21Cip1 and p27Kip1 and the decrease of CDK2. Since CQ is reported to inhibit autophagy, the catabolic process necessary for cell survival under conditions of cell starvation or stress, which is induced by cancer cells as a protective mechanism against chemotherapeutic agents, we also analyzed the induction of autophagy in HT-29. HT-29 induced autophagy in response to 5-FU, and CQ inhibited this induction, a possible mechanism of the potentiation of the anti-cancer effect of 5-FU.
Conclusion: Our findings suggest that the combination therapy with CQ should be a novel therapeutic modality to improve efficacy of 5-FU-based chemotherapy, possibly by inhibiting autophagy-dependent resistance to chemotherapy.
Figures
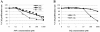
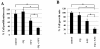

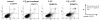

Similar articles
-
Chloroquine enhances the chemotherapeutic activity of 5-fluorouracil in a colon cancer cell line via cell cycle alteration.APMIS. 2012 Jul;120(7):597-604. doi: 10.1111/j.1600-0463.2012.02876.x. Epub 2012 Feb 11. APMIS. 2012. PMID: 22716215
-
Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study.Anticancer Drugs. 2012 Aug;23(7):675-82. doi: 10.1097/CAD.0b013e328353f8c7. Anticancer Drugs. 2012. PMID: 22561420
-
Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells.Ann Surg Oncol. 2009 Mar;16(3):761-71. doi: 10.1245/s10434-008-0260-0. Epub 2008 Dec 31. Ann Surg Oncol. 2009. PMID: 19116755
-
Repurposing Chloroquine Analogs as an Adjuvant Cancer Therapy.Recent Pat Anticancer Drug Discov. 2021;16(2):204-221. doi: 10.2174/1574892815666210106111012. Recent Pat Anticancer Drug Discov. 2021. PMID: 33413069 Review.
-
Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy.Cell Commun Signal. 2023 Feb 9;21(1):33. doi: 10.1186/s12964-023-01047-x. Cell Commun Signal. 2023. PMID: 36759799 Free PMC article. Review.
Cited by
-
Autophagy modulates the effects of bis-anthracycline WP631 on p53-deficient prostate cancer cells.J Cell Mol Med. 2015 Apr;19(4):786-98. doi: 10.1111/jcmm.12402. Epub 2015 Feb 16. J Cell Mol Med. 2015. PMID: 25689150 Free PMC article.
-
Quinacrine for extremity melanoma in a mouse model of isolated limb perfusion (ILP).Surg Today. 2015 Mar;45(3):355-62. doi: 10.1007/s00595-014-0952-y. Epub 2014 Jul 8. Surg Today. 2015. PMID: 24998594
-
Autophagy in 5-Fluorouracil Therapy in Gastrointestinal Cancer: Trends and Challenges.Chin Med J (Engl). 2016 Feb 20;129(4):456-63. doi: 10.4103/0366-6999.176069. Chin Med J (Engl). 2016. PMID: 26879020 Free PMC article. Review.
-
Dual inhibition of BDNF/TrkB and autophagy: a promising therapeutic approach for colorectal cancer.J Cell Mol Med. 2017 Oct;21(10):2610-2622. doi: 10.1111/jcmm.13181. Epub 2017 Jun 9. J Cell Mol Med. 2017. PMID: 28597984 Free PMC article.
-
Lysosomotropism depends on glucose: a chloroquine resistance mechanism.Cell Death Dis. 2017 Aug 24;8(8):e3014. doi: 10.1038/cddis.2017.416. Cell Death Dis. 2017. PMID: 28837152 Free PMC article.
References
-
- Xu R, Zhou B, Fung PC, Li X. Recent advances in the treatment of colon cancer. Histol Histopathol. 2006;21(8):867–872. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

