Structural studies of tri-functional human GART
- PMID: 20631005
- PMCID: PMC2978367
- DOI: 10.1093/nar/gkq595
Structural studies of tri-functional human GART
Abstract
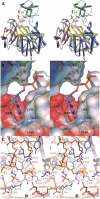
Human purine de novo synthesis pathway contains several multi-functional enzymes, one of which, tri-functional GART, contains three enzymatic activities in a single polypeptide chain. We have solved structures of two domains bearing separate catalytic functions: glycinamide ribonucleotide synthetase and aminoimidazole ribonucleotide synthetase. Structures are compared with those of homologous enzymes from prokaryotes and analyzed in terms of the catalytic mechanism. We also report small angle X-ray scattering models for the full-length protein. These models are consistent with the enzyme forming a dimer through the middle domain. The protein has an approximate seesaw geometry where terminal enzyme units display high mobility owing to flexible linker segments. This resilient seesaw shape may facilitate internal substrate/product transfer or forwarding to other enzymes in the pathway.
Figures
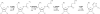
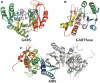


Similar articles
-
De novo purine nucleotide biosynthesis: cloning of human and avian cDNAs encoding the trifunctional glycinamide ribonucleotide synthetase-aminoimidazole ribonucleotide synthetase-glycinamide ribonucleotide transformylase by functional complementation in E. coli.Nucleic Acids Res. 1990 Nov 25;18(22):6665-72. doi: 10.1093/nar/18.22.6665. Nucleic Acids Res. 1990. PMID: 2147474 Free PMC article.
-
X-ray crystal structure of glycinamide ribonucleotide synthetase from Escherichia coli.Biochemistry. 1998 Nov 10;37(45):15647-62. doi: 10.1021/bi981405n. Biochemistry. 1998. PMID: 9843369
-
Purine biosynthesis in archaea: variations on a theme.Biol Direct. 2011 Dec 14;6:63. doi: 10.1186/1745-6150-6-63. Biol Direct. 2011. PMID: 22168471 Free PMC article.
-
A multifunctional protein possessing glycinamide ribonucleotide synthetase, glycinamide ribonucleotide transformylase, and aminoimidazole ribonucleotide synthetase activities in de novo purine biosynthesis.Biochemistry. 1985 Dec 3;24(25):7059-62. doi: 10.1021/bi00346a006. Biochemistry. 1985. PMID: 4084560
-
Carbocyclic substrates for de novo purine biosynthesis.J Biol Chem. 1991 Sep 5;266(25):16699-702. J Biol Chem. 1991. PMID: 1885598
Cited by
-
Therapeutic targeting of the mitochondrial one-carbon pathway: perspectives, pitfalls, and potential.Oncogene. 2021 Apr;40(13):2339-2354. doi: 10.1038/s41388-021-01695-8. Epub 2021 Mar 4. Oncogene. 2021. PMID: 33664451 Review.
-
A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers.PLoS Genet. 2015 Oct 16;11(10):e1005593. doi: 10.1371/journal.pgen.1005593. eCollection 2015 Oct. PLoS Genet. 2015. PMID: 26474483 Free PMC article.
-
GART Functions as a Novel Methyltransferase in the RUVBL1/β-Catenin Signaling Pathway to Promote Tumor Stemness in Colorectal Cancer.Adv Sci (Weinh). 2023 Sep;10(25):e2301264. doi: 10.1002/advs.202301264. Epub 2023 Jul 13. Adv Sci (Weinh). 2023. PMID: 37439412 Free PMC article.
-
The protection of UCK2 protein stability by GART maintains pyrimidine salvage synthesis for HCC growth under glucose limitation.Oncogene. 2025 May;44(16):1078-1092. doi: 10.1038/s41388-025-03274-7. Epub 2025 Jan 26. Oncogene. 2025. PMID: 39865175
-
A journey into the regulatory secrets of the de novo purine nucleotide biosynthesis.Front Pharmacol. 2024 Feb 20;15:1329011. doi: 10.3389/fphar.2024.1329011. eCollection 2024. Front Pharmacol. 2024. PMID: 38444943 Free PMC article. Review.
References
-
- Adam T. Purine de novo Synthesis - Mechanisms and Clinical Implications. Klin. Biochem. Metab. 2005;13:177–181.
-
- Nyhan WL. Disorders of purine and pyrimidine metabolism. Mol. Genet. Metab. 2005;86:25–33. - PubMed
-
- Hartman SC, Buchanan JM. Nucleic acids, purines, pyrimidines (nucleotide synthesis) Annu. Rev. Biochem. 1959;28:365–410. - PubMed
-
- Kappock TJ, Ealick SE, Stubbe J. Modular evolution of the purine biosynthetic pathway. Curr. Opin. Chem. Biol. 2000;4:567–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

