Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer
- PMID: 20631067
- PMCID: PMC2912978
- DOI: 10.1158/0008-5472.CAN-10-0149
Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer
Abstract
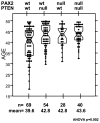
Latent endometrial carcinoma precancers are normal-appearing endometrial glands with sporadic loss of tumor suppressor gene function such as PTEN. Progression to carcinoma is inefficient and requires additional genetic damage that creates a histologic precursor lesion called endometrial intraepithelial neoplasia (EIN). In this study, we examined loss of PAX2 expression, a gene required for embryonic uterine development, during endometrial carcinogenesis. Normal proliferative, EIN, and malignant (endometrial adenocarcinoma) endometrial tissues were immunostained for PTEN and PAX2. Proliferative samples with loss of protein in at least one gland were scored as latent precancers. EIN and cancer lesions were scored by the majority pattern. Overall prevalence and topography of joint PAX2-PTEN expression loss was examined. The prevalence of PAX2 protein loss in the sequence of normal to precancer to cancer was 36%, 71%, and 77%, respectively, and for PTEN, it was 49%, 44%, and 68%, respectively. The normal endometrial prevalence of PAX2- or PTEN-deficient latent precancers was unaffected by biopsy indication, but increased significantly with age. Coincident loss of PAX2 and PTEN expression in an individual normal endometrium was seen in 21% of patients, but usually involved different glands. Coincident loss was more common in precancers (31%) and carcinoma (55%), in which case, both markers were protein null in an overlapping clonal distribution. PAX2 and PTEN protein loss occurs independently and accumulates with increasing age in latent precancers of normal premenopausal endometrium. Loss of function of both genes in an overlapping distribution characterizes the clinical emergence of a premalignant lesion which is carried forward to carcinoma.
Conflict of interest statement
Figures

References
-
- Mutter GL, Zaino RJ, Baak JPA, Bentley RC, Robboy SJ. The Benign Endometrial Hyperplasia Sequence and Endometrial Intraepithelial Neoplasia. Int J Gynecol Pathol. 2007;26:103–14. - PubMed
-
- Baak JP, Mutter GL, Robboy S, et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 2005;103(11):2304–12. - PMC - PubMed
-
- Curtis KM, Marchbanks PA, Peterson HB. Neoplasia with use of intrauterine devices. Contraception. 2007;75:S60–S69. - PubMed
-
- Grimes DA, Economy KE. Primary prevention of gynecologic cancers. Am J Obstet Gynecol. 1995;172:227–35. - PubMed
-
- Weiderpass E, Adami HO, Baron JA, et al. Use of oral contraceptives and endometrial cancer risk (Sweden) Cancer Causes Control. 1999;10:277–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

