Comparison of in-house real-time quantitative PCR to the Adenovirus R-Gene kit for determination of adenovirus load in clinical samples
- PMID: 20631100
- PMCID: PMC2937708
- DOI: 10.1128/JCM.00976-10
Comparison of in-house real-time quantitative PCR to the Adenovirus R-Gene kit for determination of adenovirus load in clinical samples
Abstract
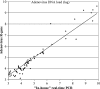
In the context of hematopoietic stem cell transplantation, adenovirus infections are associated with relevant mortality and morbidity. Detection of adenovirus DNA by quantitative PCR is the "gold standard" for these patients. A total of 150 samples, namely, 78 whole-blood, 22 cerebrospinal fluid, 24 digestive biopsy, and 26 stool samples, from 29 patients, including 24 hematopoietic stem cell transplant recipients, were tested for the detection of adenovirus using an in-house real-time quantitative PCR assay (A. Heim, C. Ebnet, G. Harste, and P. Pring-Akerblom, J. Med. Virol. 70:228-239, 2003) and the commercially available Adenovirus R-Gene kit. Adenovirus DNA was automatically isolated from whole-blood samples (Magna Pure LC system; Roche) or was manually extracted from other specimens (QIAamp; Qiagen) using the appropriate kit. The intra- and interassay reproducibilities and sensitivities were evaluated with cell culture supernatant dilutions. Of the 150 samples tested, 86 were found to be positive and 55 were found to be negative using both techniques. Nine (6%) discordant results were obtained. In most cases, discrepant results concerned samples with low viral loads. Quantitative results for all concordant positive samples were analyzed using the Spearman correlation test. A good correlation between the results of the in-house assay and those of the kit assay was obtained (r = 0.95; P < 0.001). Regarding the threshold cycle value for internal control spiked samples, none of the 150 samples tested contained a PCR inhibitor. In conclusion, a relevant correlation of results between the in-house assay and the kit assay, as well as the high-quality reproducibility and sensitivity of the kit assay, warranted its use for follow-up of hematopoietic stem cell transplantation recipients.
Figures

Similar articles
-
Comparative evaluation of a laboratory-developed real-time PCR assay and RealStar® Adenovirus PCR Kit for quantitative detection of human adenovirus.Virol J. 2018 Sep 27;15(1):149. doi: 10.1186/s12985-018-1059-7. Virol J. 2018. PMID: 30261891 Free PMC article.
-
Comparison of different approaches to quantitative adenovirus detection in stool specimens of hematopoietic stem cell transplant recipients.J Clin Virol. 2016 Dec;85:31-36. doi: 10.1016/j.jcv.2016.10.021. Epub 2016 Nov 1. J Clin Virol. 2016. PMID: 27821277
-
Diagnostic value of quantitative PCR for adenovirus detection in stool samples as compared with antigen detection and cell culture in haematopoietic stem cell transplant recipients.Clin Microbiol Infect. 2011 Nov;17(11):1674-80. doi: 10.1111/j.1469-0691.2011.03488.x. Epub 2011 Apr 11. Clin Microbiol Infect. 2011. PMID: 21481083
-
Performance evaluation of the quantitative assay Adenovirus ELITe MGB® Kit on EDTA-plasma, using the ELITe BeGenius® system.J Clin Virol. 2024 Oct;174:105722. doi: 10.1016/j.jcv.2024.105722. Epub 2024 Aug 24. J Clin Virol. 2024. PMID: 39213759
-
Broad-range PCR-electrospray ionization mass spectrometry for detection and typing of adenovirus and other opportunistic viruses in stem cell transplant patients.J Clin Microbiol. 2013 Dec;51(12):4186-92. doi: 10.1128/JCM.01978-13. Epub 2013 Oct 9. J Clin Microbiol. 2013. PMID: 24108617 Free PMC article.
Cited by
-
Immunity, safety and protection of an Adenovirus 5 prime--Modified Vaccinia virus Ankara boost subunit vaccine against Mycobacterium avium subspecies paratuberculosis infection in calves.Vet Res. 2014 Oct 29;45(1):112. doi: 10.1186/s13567-014-0112-9. Vet Res. 2014. PMID: 25480162 Free PMC article.
-
Development of a Rapid Immuno-Based Screening Assay for the Detection of Adenovirus in Eye Infections.ACS Omega. 2022 May 19;7(21):17555-17562. doi: 10.1021/acsomega.1c07022. eCollection 2022 May 31. ACS Omega. 2022. PMID: 35664618 Free PMC article.
-
Intestinal HAdV Infection: Tissue Specificity, Persistence, and Implications for Antiviral Therapy.Viruses. 2019 Aug 30;11(9):804. doi: 10.3390/v11090804. Viruses. 2019. PMID: 31480296 Free PMC article. Review.
-
How I treat adenovirus in hematopoietic stem cell transplant recipients.Blood. 2010 Dec 16;116(25):5476-85. doi: 10.1182/blood-2010-04-259291. Epub 2010 Sep 13. Blood. 2010. PMID: 20837781 Free PMC article. Review.
-
A cost effective real-time PCR for the detection of adenovirus from viral swabs.Virol J. 2013 Jun 7;10:184. doi: 10.1186/1743-422X-10-184. Virol J. 2013. PMID: 23758993 Free PMC article.
References
-
- Aissi-Rothe, L., V. Decot, V. Venard, H. Jeulin, A. Salmon, L. Clement, A. Kennel, C. Mathieu, J. H. Dalle, G. Rauser, C. Cambouris, M. de Carvalho, J. F. Stoltz, P. Bordigoni, and D. Bensoussan. 2010. Rapid generation of full clinical-grade human antiadenovirus cytotoxic T cells for adoptive immunotherapy. J. Immunother. 33:414-424. - PubMed
-
- Bordigoni, P., A. S. Carret, V. Venard, F. Witz, and A. Le Faou. 2001. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 32:1290-1297. - PubMed
-
- Chakrabarti, S., K. E. Collingham, R. H. Stevens, D. Pillay, C. D. Fegan, and D. W. Milligan. 2000. Isolation of viruses from stools in stem cell transplant recipients: a prospective surveillance study. Bone Marrow Transplant. 25:277-282. - PubMed
-
- de Mezerville, M. H., R. Tellier, S. Richardson, D. Hebert, J. Doyle, and U. Allen. 2006. Adenoviral infections in pediatric transplant recipients: a hospital-based study. Pediatr. Infect. Dis. J. 25:815-818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

