Two overlapping domains of a lyssavirus matrix protein that acts on different cell death pathways
- PMID: 20631119
- PMCID: PMC2937802
- DOI: 10.1128/JVI.00761-10
Two overlapping domains of a lyssavirus matrix protein that acts on different cell death pathways
Abstract
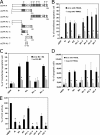
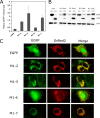
The lyssavirus matrix (M) protein induces apoptosis. The regions of the M protein that are essential for triggering cell death pathways are not yet clearly defined. We therefore compared the M proteins from two viruses that have contrasting characteristics in terms of cellular apoptosis: a genotype 3 lyssavirus, Mokola virus (MOK), and a genotype 1 rabies virus isolated from a dog from Thailand (THA). We identified a 20-amino-acid fragment (corresponding to positions 67 to 86) that retained the cell death activities of the full-length M protein from MOK via both the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and inhibition of cytochrome c oxidase (CcO) activity. We found that the amino acids at positions 77 and 81 have an essential role in triggering these two cell death pathways. Directed mutagenesis demonstrated that the amino acid at position 77 affects CcO activity, whereas the amino acid at position 81 affects TRAIL-dependent apoptosis. Mutations in the full-length M protein that compromised induction of either of these two pathways resulted in delayed apoptosis compared with the time to apoptosis for the nonmutated control.
Figures
Similar articles
-
Mitochondrial dysfunction in lyssavirus-induced apoptosis.J Virol. 2008 May;82(10):4774-84. doi: 10.1128/JVI.02651-07. Epub 2008 Mar 5. J Virol. 2008. PMID: 18321977 Free PMC article.
-
Intergenotypic replacement of lyssavirus matrix proteins demonstrates the role of lyssavirus M proteins in intracellular virus accumulation.J Virol. 2010 Feb;84(4):1816-27. doi: 10.1128/JVI.01665-09. Epub 2009 Dec 2. J Virol. 2010. PMID: 19955305 Free PMC article.
-
Lyssavirus matrix protein induces apoptosis by a TRAIL-dependent mechanism involving caspase-8 activation.J Virol. 2004 Jun;78(12):6543-55. doi: 10.1128/JVI.78.12.6543-6555.2004. J Virol. 2004. PMID: 15163747 Free PMC article.
-
Human rabies due to lyssavirus infection of bat origin.Vet Microbiol. 2010 May 19;142(3-4):151-9. doi: 10.1016/j.vetmic.2010.02.001. Epub 2010 Feb 6. Vet Microbiol. 2010. PMID: 20188498 Review.
-
[Rabies and other lyssaviruses].Uirusu. 2004 Dec;54(2):213-22. doi: 10.2222/jsv.54.213. Uirusu. 2004. PMID: 15745159 Review. Japanese.
Cited by
-
Rabies virus phosphoprotein interacts with mitochondrial Complex I and induces mitochondrial dysfunction and oxidative stress.J Neurovirol. 2015 Aug;21(4):370-82. doi: 10.1007/s13365-015-0320-8. Epub 2015 Feb 20. J Neurovirol. 2015. PMID: 25698500
-
Purification and characterization of a recombinant bacteriophytochrome of Xanthomonas oryzae pathovar oryzae.Protein J. 2011 Feb;30(2):124-31. doi: 10.1007/s10930-011-9312-6. Protein J. 2011. PMID: 21318274
-
Regulation of NF-κB by the p105-ABIN2-TPL2 complex and RelAp43 during rabies virus infection.PLoS Pathog. 2017 Oct 30;13(10):e1006697. doi: 10.1371/journal.ppat.1006697. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29084252 Free PMC article.
-
Wild-type rabies virus induces autophagy in human and mouse neuroblastoma cell lines.Autophagy. 2016 Oct 2;12(10):1704-1720. doi: 10.1080/15548627.2016.1196315. Epub 2016 Jul 27. Autophagy. 2016. PMID: 27463027 Free PMC article.
-
Systems Biomedicine of Rabies Delineates the Affected Signaling Pathways.Front Microbiol. 2016 Nov 7;7:1688. doi: 10.3389/fmicb.2016.01688. eCollection 2016. Front Microbiol. 2016. PMID: 27872612 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

