Development of a targeted gene vector platform based on simian adenovirus serotype 24
- PMID: 20631120
- PMCID: PMC2937776
- DOI: 10.1128/JVI.02425-09
Development of a targeted gene vector platform based on simian adenovirus serotype 24
Abstract
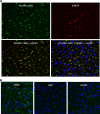
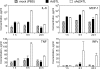
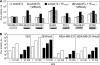
Efforts to develop adenovirus vectors suitable for genetic interventions in humans have identified three major limitations of the most frequently used vector prototype, human adenovirus serotype 5 (Ad5). These limitations--widespread preexisting anti-Ad5 immunity in humans, the high rate of transduction of normal nontarget tissues, and the lack of target-specific gene delivery--justify the exploration of other Ad serotypes as vector prototypes. In this paper, we describe the development of an alternative vector platform using simian Ad serotype 24 (sAd24). We found that sAd24 virions formed unstable complexes with blood coagulation factor X and, because of that, transduced the liver and other organs at low levels when administered intravenously. The overall pattern of biodistribution of sAd24 particles was similar, however, to that of Ad5, and the intravenously injected sAd24 was cleared by Kupffer cells, leading to their depletion. We modified the virus's fiber protein to design a Her2-specific derivative of sAd24 capable of infecting target human tumor cells in vitro. In the presence of neutralizing anti-Ad5 antibodies, Her2-mediated infection with targeted sAd24 compared favorably to that with the Ad5-derived vector. When used to target Her2-expressing tumors in animals, this fiber-modified vector achieved a higher level of gene transfer to metastasis-containing murine lungs than to tumor-free lungs. In aggregate, these studies provide important insights into sAd24 biology, identify its advantages and limitations as a vector prototype, and are thus essential for further development of an sAd24-based gene delivery platform.
Figures

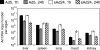




References
-
- Alauddin, M. M., P. S. Conti, and J. D. Fissekis. 2003. A general synthesis of 2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-d-arabinofuranosyluracil and its 5-substituted nucleosides. J. Label. Comp. Radiopharm. 46:285-289.
-
- Alba, R., A. C. Bradshaw, A. L. Parker, D. Bhella, S. N. Waddington, S. A. Nicklin, N. van Rooijen, J. Custers, J. Goudsmit, D. H. Barouch, J. H. McVey, and A. H. Baker. 2009. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood 114:965-971. - PMC - PubMed
-
- Appledorn, D. M., A. Kiang, A. McBride, H. Jiang, S. Seregin, J. M. Scott, R. Stringer, Y. Kousa, M. Hoban, M. M. Frank, and A. Amalfitano. 2008. Wild-type adenoviruses from groups A-F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 15:885-901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

