Mitochondrial morphology and cardiovascular disease
- PMID: 20631158
- PMCID: PMC2936127
- DOI: 10.1093/cvr/cvq237
Mitochondrial morphology and cardiovascular disease
Abstract
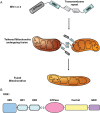
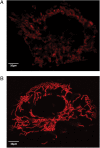
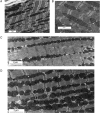
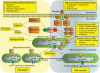
Mitochondria are dynamic and are able to interchange their morphology between elongated interconnected mitochondrial networks and a fragmented disconnected arrangement by the processes of mitochondrial fusion and fission, respectively. Changes in mitochondrial morphology are regulated by the mitochondrial fusion proteins (mitofusins 1 and 2, and optic atrophy 1) and the mitochondrial fission proteins (dynamin-related peptide 1 and mitochondrial fission protein 1) and have been implicated in a variety of biological processes including embryonic development, metabolism, apoptosis, and autophagy, although the majority of studies have been largely confined to non-cardiac cells. Despite the unique arrangement of mitochondria in the adult heart, emerging data suggest that changes in mitochondrial morphology may be relevant to various aspects of cardiovascular biology-these include cardiac development, the response to ischaemia-reperfusion injury, heart failure, diabetes mellitus, and apoptosis. Interestingly, the machinery required for altering mitochondrial shape in terms of the mitochondrial fusion and fission proteins are all present in the adult heart, but their physiological function remains unclear. In this article, we review the current developments in this exciting new field of mitochondrial biology, the implications for cardiovascular physiology, and the potential for discovering novel therapeutic strategies for treating cardiovascular disease.
Figures


References
-
- Hausenloy DJ, Scorrano L. Targeting cell death. Clin Pharmacol Ther. 2007;82:370–373. doi:10.1038/sj.clpt.6100352. - DOI - PubMed
-
- Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi:10.1152/physrev.00030.2008. - DOI - PubMed
-
- Benda C. Ueber dier Spermatogenese de Verbebraten und hoherer Evertebraten, II. Theil: Die Histiogenese der Spermien. Arch Anat Physiol. 1898;73:393–398.
-
- Dimmer KS, Scorrano L. (De)constructing mitochondria: what for? Physiology (Bethesda) 2006;21:233–241. - PubMed
-
- Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–358. doi:10.1083/jcb.143.2.351. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

