Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment
- PMID: 20631294
- PMCID: PMC2944434
- DOI: 10.1152/ajpregu.00797.2009
Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment
Abstract
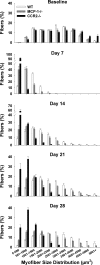
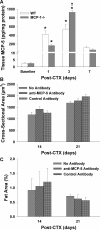
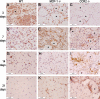
Muscle regeneration requires CC chemokine receptor 2 (CCR2) expression on bone marrow-derived cells; macrophages are a prominent CCR2-expressing cell in this process. CCR2-/- mice have severe impairments in angiogenesis, macrophage recruitment, and skeletal muscle regeneration following cardiotoxin (CTX)-induced injury. However, multiple chemokines activate CCR2, including monocyte chemotactic proteins (MCP)-1, -3, and -5. We hypothesized that MCP-1 is the chemokine ligand that mediates the impairments present in CCR2-/- mice. We examined muscle regeneration, capillary density, and cellular recruitment in MCP-1-/- and CCR2-/- mice following injury. Muscle regeneration and adipocyte accumulation, but not capillary density, were significantly impaired in MCP-1-/- compared with wild-type (WT) mice; however, muscle regeneration and adipocyte accumulation impairments were not as severe as observed in CCR2-/- mice. Although tissue levels of MCP-5 were elevated in MCP-1-/- mice compared with WT, the administration of MCP-5 neutralizing antibody did not alter muscle regeneration in MCP-1-/- mice. While neutrophil accumulation after injury was similar in all three mouse strains, macrophage recruitment was highest in WT mice, intermediate in MCP-1-/- mice, and severely impaired in CCR2-/- mice. In conclusion, while the absence of MCP-1 resulted in impaired macrophage recruitment and muscle regeneration, MCP-1-/- mice exhibit an intermediate phenotype compared with CCR2-/- mice. Intermediate macrophage recruitment in MCP-1-/- mice was associated with similar capillary density to WT, suggesting that fewer macrophages may be needed to restore angiogenesis vs. muscle regeneration. Finally, other chemokines, in addition to MCP-1 and MCP-5, may activate CCR2-dependent regenerative processes resulting in an intermediate phenotype in MCP-1-/- mice.
Figures




References
-
- Asakura A. Stem cells in adult skeletal muscle. Trends Cardiovasc Med 13: 123–128, 2003 - PubMed
-
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004 - PubMed
-
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354: 610–621, 2006 - PubMed
-
- Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res 95: 858–866, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

