Comparison of immunologic assays for detecting immune responses in HIV immunotherapeutic studies: AIDS Clinical Trials Group Trial A5181
- PMID: 20631337
- PMCID: PMC2944467
- DOI: 10.1128/CVI.00498-09
Comparison of immunologic assays for detecting immune responses in HIV immunotherapeutic studies: AIDS Clinical Trials Group Trial A5181
Abstract
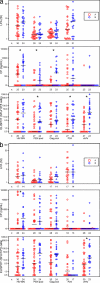
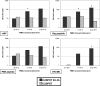
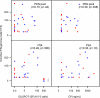
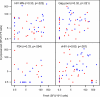
This study was designed to evaluate which of several T-cell-specific, immune response assays are the most relevant in measuring the key characteristics of an effective immune response to HIV-1. Using 5 HIV-1 antigens as stimulants, we assessed lymphocyte proliferation, supernatant gamma interferon (IFN-gamma) cytokine production (CP), single-cell IFN-gamma production by enzyme-linked immunospot (ELISPOT) assay, with and without Epstein-Barr virus-transformed B-lymphoblastoid cell lines (B-LCLs), and intracellular cytokine production (ICC) for IFN-gamma and interleukin 2 (IL-2) by flow cytometry. We used these to compare specimens from HIV-1-infected subjects who were virally suppressed with a stable antiretroviral therapy (ART) regimen (group A) with specimens from subjects not on ART but with HIV-1 viremia of <3,000 copies/ml (group B). The lymphocyte proliferation assay (LPA) did not significantly differentiate between the two groups. Using fresh peripheral blood mononuclear cells (PBMCs), the CP and ELISPOT assays for IFN-gamma detected the greatest differences between the two groups, specific for three of the five HIV-1 antigens, whereas significant differences were seen only in response to one antigen when cryopreserved cells were used. The strongest correlations were seen between the CP and ELISPOT assays. The ELISPOT B-LCL assay showed a cell concentration-dependent increase in IFN-gamma production compared to that shown by the standard ELISPOT assay but did not differentiate between the groups. In the ICC assay, greater numbers of IFN-gamma-producing T cells were seen in group B, and little or no detectable IL-2 production was seen in both groups. These studies highlight complexities of immunologic monitoring of T-cell responses in multisite clinical trials in HIV infection and outline considerations for optimizing these efforts.
Figures
Similar articles
-
Assessment of immune function by lymphoproliferation underestimates lymphocyte functional capacity in HIV patients treated with highly active antiretroviral therapy.AIDS Res Hum Retroviruses. 2000 Dec 10;16(18):1991-6. doi: 10.1089/088922200750054729. AIDS Res Hum Retroviruses. 2000. PMID: 11153082
-
Interferon gamma (IFN-γ) negative CD4+ and CD8+ T-cells can produce immune mediators in response to viral antigens.Vaccine. 2019 Jan 3;37(1):113-122. doi: 10.1016/j.vaccine.2018.11.024. Epub 2018 Nov 17. Vaccine. 2019. PMID: 30459072 Free PMC article.
-
Human immunodeficiency virus type I-specific CD8+ T cell subset abnormalities in chronic infection persist through effective antiretroviral therapy.BMC Infect Dis. 2010 May 25;10:129. doi: 10.1186/1471-2334-10-129. BMC Infect Dis. 2010. PMID: 20500844 Free PMC article.
-
Establishment and maintenance of a PBMC repository for functional cellular studies in support of clinical vaccine trials.J Immunol Methods. 2014 Jul;409:107-16. doi: 10.1016/j.jim.2014.04.005. Epub 2014 Apr 29. J Immunol Methods. 2014. PMID: 24787274 Free PMC article.
-
Rapid qualitative and quantitative analysis of T-cell responses in HIV-1-infected individuals receiving successful HAART and HIV-1 sero-negative controls: concomitant assessment of perforin, IFN-gamma and IL-4 secretion.J Immunol Methods. 2006 Jan 20;308(1-2):216-30. doi: 10.1016/j.jim.2005.11.005. Epub 2005 Dec 19. J Immunol Methods. 2006. PMID: 16388819
Cited by
-
Dendritic cells reveal a broad range of MHC class I epitopes for HIV-1 in persons with suppressed viral load on antiretroviral therapy.PLoS One. 2010 Sep 23;5(9):e12936. doi: 10.1371/journal.pone.0012936. PLoS One. 2010. PMID: 20886040 Free PMC article.
-
Beyond Efficacy: Ensuring Safety in Peptide Therapeutics through Immunogenicity Assessment.J Pept Sci. 2025 Jun;31(6):e70016. doi: 10.1002/psc.70016. J Pept Sci. 2025. PMID: 40256940 Free PMC article. Review.
-
Overcoming immunogenicity issues of HIV p24 antigen by the use of innovative nanostructured lipid carriers as delivery systems: evidences in mice and non-human primates.NPJ Vaccines. 2018 Oct 1;3:46. doi: 10.1038/s41541-018-0086-0. eCollection 2018. NPJ Vaccines. 2018. PMID: 30302284 Free PMC article.
-
Preserving HIV-specific T cell responses: does timing of antiretroviral therapy help?Curr Opin HIV AIDS. 2015 Jan;10(1):55-60. doi: 10.1097/COH.0000000000000124. Curr Opin HIV AIDS. 2015. PMID: 25389805 Free PMC article. Review.
-
An open-label, multiple ascending dose study of the anti-CTLA-4 antibody ipilimumab in viremic HIV patients.PLoS One. 2018 Jun 7;13(6):e0198158. doi: 10.1371/journal.pone.0198158. eCollection 2018. PLoS One. 2018. PMID: 29879143 Free PMC article. Clinical Trial.
References
-
- Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Elridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. - PMC - PubMed
-
- Best, A., G. Hidalgo, K. Mitchell, and J. R. Yannelli. 2007. Issues concerning the large scale cryopreservation of peripheral blood mononuclear cells (PBMC) for immunotherapy trials. Cryobiology 54:294-297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI69494/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- AI54907/AI/NIAID NIH HHS/United States
- P30 AI054907/AI/NIAID NIH HHS/United States
- AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- P30 AI073961/AI/NIAID NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- U01 AI027675/AI/NIAID NIH HHS/United States
- AI68634/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- AI038858/AI/NIAID NIH HHS/United States
- AI69501/AI/NIAID NIH HHS/United States
- AI27675/AI/NIAID NIH HHS/United States
- AI69477/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- AI68638/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- AI69450/AI/NIAID NIH HHS/United States
- AI73961/AI/NIAID NIH HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- AI069471/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- RR25780/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

