Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells
- PMID: 20631379
- PMCID: PMC2981541
- DOI: 10.1182/blood-2010-01-265041
Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells
Abstract
Adoptive T-cell therapy with anti-CD19 chimeric antigen receptor (CAR)-expressing T cells is a new approach for treating advanced B-cell malignancies. To evaluate anti-CD19-CAR-transduced T cells in a murine model of adoptive T-cell therapy, we developed a CAR that specifically recognized murine CD19. We used T cells that were retrovirally transduced with this CAR to treat mice bearing a syngeneic lymphoma that naturally expressed the self-antigen murine CD19. One infusion of anti-CD19-CAR-transduced T cells completely eliminated normal B cells from mice for at least 143 days. Anti-CD19-CAR-transduced T cells eradicated intraperitoneally injected lymphoma cells and large subcutaneous lymphoma masses. The antilymphoma efficacy of anti-CD19-CAR-transduced T cells was critically dependent on irradiation of mice before anti-CD19-CAR-transduced T-cell infusion. Anti-CD19-CAR-transduced T cells had superior antilymphoma efficacy compared with the anti-CD19 monoclonal antibody from which the anti-CD19 CAR was derived. Our results demonstrated impressive antilymphoma activity and profound destruction of normal B cells caused by anti-CD19-CAR-transduced T cells in a clinically relevant murine model.
Figures
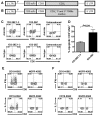
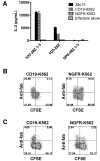


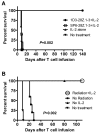
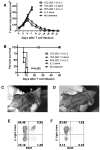

References
-
- Kershaw MH, Teng MWL, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5(12):928–940. - PubMed
-
- Rossig C, Bar A, Pscherer S, et al. Target antigen expression on a professional antigen-presenting cell induces superior proliferative antitumor T-cell responses via chimeric T-cell receptors. J Immunother. 2006;29(1):21–31. - PubMed
-
- Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells [see comment]. Leukemia. 2006;20(10):1819–1828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

