Regulation of cAMP by phosphodiesterases in erythrocytes
- PMID: 20631411
- PMCID: PMC2922877
- DOI: 10.1016/s1734-1140(10)70303-0
Regulation of cAMP by phosphodiesterases in erythrocytes
Abstract
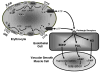
The erythrocyte, a cell responsible for carrying and delivering oxygen in the body, has often been regarded as simply a vehicle for the circulation of hemoglobin. However, it has become evident that this cell also participates in the regulation of vascular caliber in the microcirculation via release of the potent vasodilator, adenosine triphosphate (ATP). The regulated release of ATP from erythrocytes occurs via a defined signaling pathway and requires increases in cyclic 3',5'- adenosine monophosphate (cAMP). It is well recognized that cAMP is a critical second messenger in diverse signaling pathways. In all cells increases in cAMP are localized and regulated by the activity of phosphodiesterases (PDEs). In erythrocytes activation of either beta adrenergic receptors (beta(2)AR) or the prostacyclin receptor (IPR) results in increases in cAMP and ATP release. Receptor-mediated increases in cAMP are tightly regulated by distinct PDEs associated with each signaling pathway as shown by the finding that selective inhibitors of the PDEs localized to each pathway potentiate both increases in cAMP and ATP release. Here we review the profile of PDEs identified in erythrocytes, their association with specific signaling pathways and their role in the regulation of ATP release from these cells. Understanding the contribution of PDEs to the control of ATP release from erythrocytes identifies this cell as a potential target for the development of drugs for the treatment of vascular disease.
Figures

References
-
- Adderley SP, Dufaux EA, Sridharan M, Bowles EA, Hanson MS, Stephenson AH, Ellsworth ML, Sprague RS. Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans. Am J Physiol Heart Circ Physiol. 2009;296:H1617–1624. - PMC - PubMed
-
- Babu CR, Azhar S, Krishna Murti CR. Loss of epinephrine stimulated synthesis of cyclic adenosine 3':5' monophosphate during maturation of rabbit and human reticulocytes. Med Biol. 1975;53:148–155. - PubMed
-
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. - PubMed
-
- Baillie GS. Compartmentalized signaling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. The FEBS journal. 2009;276:1790–1799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
