Pathogenesis of Graves' orbitopathy: a 2010 update
- PMID: 20631493
- PMCID: PMC6779038
- DOI: 10.1007/BF03346614
Pathogenesis of Graves' orbitopathy: a 2010 update
Abstract
The most important of the extra-thyroidal manifestations of Graves' disease, Graves' orbitopathy (GO), remains a vexing clinical problem. Treatment of severe active disease has been limited to steroids or radiotherapy. In the relatively rare case where vision is threatened, emergent decompression surgery can be performed. The proptosis, motility, or cosmetic concerns associated with stable GO are commonly remedied with surgical intervention. Substantial obstacles have prevented the development of specific medical therapies for GO, in large part resulting from poor understanding of disease pathogenesis and the absence of preclinical animal models. Fundamental aspects of GO's etiology have been uncovered from studies based in cell culture, extensive analysis of blood constituents, and detailed examination of orbital contents collected at the time of surgical intervention. Many of the published reports resulting from these studies are descriptive and all have failed to yield unifying concepts that integrate the anatomically divergent manifestations of Graves' disease. This brief review covers recent findings of several research groups. While major breakthroughs continue to occur in closely related autoimmune diseases, progress in identifying the pathogenic mechanisms relevant to GO has been limited. As emerging insights into human autoimmunity becomes applied to the study of Graves' disease, we anticipate that improved therapeutic strategies will find their way to our patients with GO.
Figures

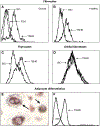
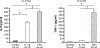
References
-
- Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the Pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev 2003, 24: 802–35. - PubMed
-
- Parmentier M, Libert F, Maenhaut C, et al. Molecular cloning of the thyrotropin receptor. Science 1989, 246: 1620–2. - PubMed
-
- McKenzie JM. Humoral factors in the pathogenesis of Graves’ disease. Physiol Rev 1968, 48: 252–309. - PubMed
-
- Feliciello A, Porcellini A, Ciullo I, Bonavolontà G, Avvedimento EV, Fenzi G.. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet 1993, 342: 337–8. - PubMed
-
- Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid 1993, 3: 297–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

