How neurons generate behavior in a hatchling amphibian tadpole: an outline
- PMID: 20631854
- PMCID: PMC2903309
- DOI: 10.3389/fnbeh.2010.00016
How neurons generate behavior in a hatchling amphibian tadpole: an outline
Abstract
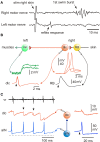
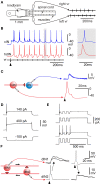
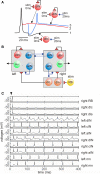
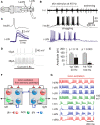
Adult nervous systems are so complex that understanding how they produce behavior remains a real challenge. We chose to study hatchling Xenopus tadpoles where behavior is controlled by a few thousand neurons but there is a very limited number of types of neuron. Young tadpoles can flex, swim away, adjust their trajectory, speed-up and slow-down, stop when they contact support and struggle when grasped. They are sensitive to touch, pressure, noxious stimuli, light intensity and water currents. Using whole-cell recording has led to rapid progress in understanding central networks controlling behavior. Our methods are illustrated by an analysis of the flexion reflex to skin touch. We then define the seven types of neuron that allow the tadpole to swim when the skin is touched and use paired recordings to investigate neuron properties, synaptic connections and activity patterns. Proposals on how the swim network operates are evaluated by experiment and network modeling. We then examine GABAergic inhibitory pathways that control swimming but also produce tonic inhibition to reduce responsiveness when the tadpole is at rest. Finally, we analyze the strong alternating struggling movements the tadpole makes when grasped. We show that the mechanisms for rhythm generation here are very different to those during swimming. Although much remains to be explained, study of this simple vertebrate has uncovered basic principles about the function and organization of vertebrate nervous systems.
Keywords: Xenopus; pattern generation; reflex; spinal interneurons; tonic inhibition.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

