Thrombospondin-1 (TSP-1) Stimulates Expression of Integrin alpha6 in Human Breast Carcinoma Cells: A Downstream Modulator of TSP-1-Induced Cellular Adhesion
- PMID: 20631908
- PMCID: PMC2902750
- DOI: 10.1155/2010/645376
Thrombospondin-1 (TSP-1) Stimulates Expression of Integrin alpha6 in Human Breast Carcinoma Cells: A Downstream Modulator of TSP-1-Induced Cellular Adhesion
Abstract
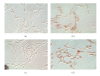
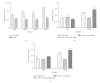
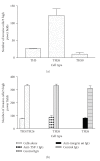
Thrombospondin-1 (TSP-1) is involved in a variety of different cellular processes including cell adhesion, tumor progression, and angiogenesis. This paper reports the novel finding that TSP-1 upregulates integrin alpha6 subunit in human keratinocytes and human breast cancer cells resulting in increased cell adhesion and tumor cell invasion. The effect of TSP-1 on alpha6 subunit expression was examined in human keratinocytes and breast adenocarcinoma cell lines (MDA-MB-231) treated with TSP-1 and in TSP-1 stably transfected breast cancer cells. TSP-1 upregulated alpha6 message and protein in these cells as revealed by differential display, Northern and Western blot analysis and immunohistochemical localization studies. The increased expression of alpha6 was shown to mediate adhesion and invasion of these cells to laminin, a major component of the basement membrane and extracellular matrix (ECM). These data suggest that TSP-1 plays an integral role in the attachment of cells to the ECM facilitating cell motility and angiogenesis.
Figures


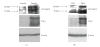

Similar articles
-
Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment.Clin Exp Metastasis. 2005;22(5):391-402. doi: 10.1007/s10585-005-1262-y. Clin Exp Metastasis. 2005. PMID: 16283482
-
Scatter factor binds to thrombospondin and other extracellular matrix components.Am J Pathol. 1996 Sep;149(3):805-19. Am J Pathol. 1996. PMID: 8780385 Free PMC article.
-
Involvement of thrombospondin in the adherence of human breast-adenocarcinoma cells: a possible role in the metastatic process.Int J Cancer. 1993 Sep 30;55(3):471-7. doi: 10.1002/ijc.2910550325. Int J Cancer. 1993. PMID: 8375932
-
Thrombospondin-1 Signaling Through the Calreticulin/LDL Receptor Related Protein 1 Axis: Functions and Possible Roles in Glaucoma.Front Cell Dev Biol. 2022 May 27;10:898772. doi: 10.3389/fcell.2022.898772. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35693935 Free PMC article. Review.
-
Expression of thrombospondin-1 in cancer: a role in tumor progression.Proc Soc Exp Biol Med. 1996 Jul;212(3):199-207. doi: 10.3181/00379727-212-44008. Proc Soc Exp Biol Med. 1996. PMID: 8677265 Review.
Cited by
-
Bothrops Jararaca Snake Venom Modulates Key Cancer-Related Proteins in Breast Tumor Cell Lines.Toxins (Basel). 2021 Jul 25;13(8):519. doi: 10.3390/toxins13080519. Toxins (Basel). 2021. PMID: 34437390 Free PMC article.
-
Thrombospondin-1 is a multifaceted player in tumor progression.Oncotarget. 2017 Jul 11;8(48):84546-84558. doi: 10.18632/oncotarget.19165. eCollection 2017 Oct 13. Oncotarget. 2017. PMID: 29137447 Free PMC article. Review.
-
Gene expression profiles of the NCI-60 human tumor cell lines define molecular interaction networks governing cell migration processes.PLoS One. 2012;7(5):e35716. doi: 10.1371/journal.pone.0035716. Epub 2012 May 3. PLoS One. 2012. PMID: 22570691 Free PMC article.
-
Critical role of thrombospondin-1 in promoting intestinal mucosal wound repair.JCI Insight. 2024 Jul 30;9(17):e180608. doi: 10.1172/jci.insight.180608. JCI Insight. 2024. PMID: 39078701 Free PMC article.
-
A proteomics platform combining depletion, multi-lectin affinity chromatography (M-LAC), and isoelectric focusing to study the breast cancer proteome.Anal Chem. 2011 Jun 15;83(12):4845-54. doi: 10.1021/ac2002802. Epub 2011 May 23. Anal Chem. 2011. PMID: 21513341 Free PMC article.
References
-
- Albo D, Berger DH, Wang TN, Hu X, Rothman V, Tuszynski GP. Thrombospondin-1 and transforming growth factor-beta1 promote breast tumor cell invasion through up-regulation of the plasminogen/plasmin system. Surgery. 1997;122(2):493–500. - PubMed
-
- Heino J. Biology of tumor cell invasion: interplay of cell adhesion and matrix degradation. International Journal of Cancer. 1996;65(6):717–722. - PubMed
-
- Song S-Y, Nomizu M, Yamada Y, Kleinman HK. Liver metastasis formation by laminin-1 peptide (LQVQLSIR)-adhesion selected B16-F10 melanoma cells. International Journal of Cancer. 1997;71(3):436–441. - PubMed
-
- Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. The Journal of Biological Chemistry. 1998;273(6):3604–3610. - PubMed
-
- Lawler JW, Slayter HS, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. The Journal of Biological Chemistry. 1978;253(23):8609–8616. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

