Constructing multiply substituted arenes using sequential palladium(II)-catalyzed C-H olefination
- PMID: 20632344
- PMCID: PMC3269767
- DOI: 10.1002/anie.201002077
Constructing multiply substituted arenes using sequential palladium(II)-catalyzed C-H olefination
Abstract
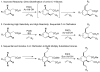
Complementary catalytic systems have been developed in which the reactivity/selectivity balance in Pd(II)-catalyzed ortho-C–H olefination can be modulated through ligand control. This allows for sequential C–H functionalization for the rapid preparation of 1,2,3-trisubstituted arenes. Additionally, a rare example of iterative C–H activation, in which a newly installed functional group directs subsequent C–H activation has been demonstrated.
Figures



Similar articles
-
Pd-catalyzed C-H olefination of (hetero)arenes by using saturated ketones as an olefin source.Angew Chem Int Ed Engl. 2013 Jan 21;52(4):1299-303. doi: 10.1002/anie.201208627. Epub 2012 Dec 7. Angew Chem Int Ed Engl. 2013. PMID: 23225575 No abstract available.
-
Pd(II)-catalyzed olefination of sp3 C-H bonds.J Am Chem Soc. 2010 Mar 24;132(11):3680-1. doi: 10.1021/ja1010866. J Am Chem Soc. 2010. PMID: 20187642 Free PMC article.
-
Ether-directed ortho-C-H olefination with a palladium(II)/monoprotected amino acid catalyst.Angew Chem Int Ed Engl. 2013 Jan 21;52(4):1245-7. doi: 10.1002/anie.201207770. Epub 2012 Dec 13. Angew Chem Int Ed Engl. 2013. PMID: 23239120 Free PMC article.
-
Palladium-catalyzed allylic C-H bond functionalization of olefins.Top Curr Chem. 2010;292:195-209. doi: 10.1007/128_2009_16. Top Curr Chem. 2010. PMID: 21500407 Review.
-
C-C, C-O, C-N bond formation on sp2 carbon by Pd(II)-catalyzed reactions involving oxidant agents.Chem Rev. 2007 Nov;107(11):5318-65. doi: 10.1021/cr068006f. Epub 2007 Nov 1. Chem Rev. 2007. PMID: 17973536 Review. No abstract available.
Cited by
-
Highly convergent total synthesis of (+)-lithospermic acid via a late-stage intermolecular C-H olefination.J Am Chem Soc. 2011 Apr 20;133(15):5767-9. doi: 10.1021/ja2010225. Epub 2011 Mar 28. J Am Chem Soc. 2011. PMID: 21443224 Free PMC article.
-
Palladium mono-N-protected amino acid complexes: experimental validation of the ligand cooperation model in C-H activation.Chem Sci. 2023 May 26;14(24):6688-6694. doi: 10.1039/d3sc02076b. eCollection 2023 Jun 21. Chem Sci. 2023. PMID: 37350841 Free PMC article.
-
Rh(III)-catalyzed oxidative coupling of unactivated alkenes via C-H activation.Org Lett. 2011 Feb 4;13(3):540-2. doi: 10.1021/ol102890k. Epub 2010 Dec 22. Org Lett. 2011. PMID: 21175143 Free PMC article.
-
C-H Glycosylation of Native Carboxylic Acids: Discovery of Antidiabetic SGLT-2 Inhibitors.ACS Cent Sci. 2023 Jun 9;9(6):1129-1139. doi: 10.1021/acscentsci.3c00201. eCollection 2023 Jun 28. ACS Cent Sci. 2023. PMID: 37396867 Free PMC article.
-
Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes Using Molecular Oxygen as the Sole Oxidant Enabled by Quaternary Ammonium Salt.Molecules. 2021 Sep 2;26(17):5329. doi: 10.3390/molecules26175329. Molecules. 2021. PMID: 34500762 Free PMC article.
References
-
- Burroughes JH, Bradley DDC, Brown AR, Marks RN, Mackay K. Nature. 1990;347:539.
-
-
For reviews of electroluminescent conjugated polymers, see: Kraft A, Grimsdale AC, Holmes AB. Angew Chem, Int Ed. 1998;37:402.Grimsdale AC, Chan KL, Martin RE, Jokisz PG, Holmes AB. Chem Rev. 2009;109:897.
-
-
- Mizoroki T, Mori K, Ozaki A. Bull Chem Soc Jpn. 1971;44:581.
- Heck RF, Nolley JP., Jr J Org Chem. 1972;37:2320.
-
- Plevyak JE, Heck RF. J Org Chem. 1978;43:2454.
-
-
For examples of differential Mizoroki–Heck reactions to construct unsymmetrical divinylbenzenes, see: Plevyak JE, Dickerson JE, Heck RF. J Org Chem. 1979;44:4078.Spencer AJ. J Organomet Chem. 1984;265:323.Tao WJ, Nesbitt S, Heck RF. J Org Chem. 1990;55:63.Carlström AS, Frejd T. J Org Chem. 1991;56:1289.Sengupta S, Sadhukhan SK. Tetrahedron Lett. 1998;39:715.Wang C, Tan LS, He JP, Hu HW, Xu JH. Synth Commun. 2003;33:773.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

