MRI quantification of non-Gaussian water diffusion by kurtosis analysis
- PMID: 20632416
- PMCID: PMC2997680
- DOI: 10.1002/nbm.1518
MRI quantification of non-Gaussian water diffusion by kurtosis analysis
Abstract
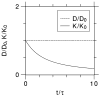
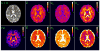
Quantification of non-Gaussianity for water diffusion in brain by means of diffusional kurtosis imaging (DKI) is reviewed. Diffusional non-Gaussianity is a consequence of tissue structure that creates diffusion barriers and compartments. The degree of non-Gaussianity is conveniently quantified by the diffusional kurtosis and derivative metrics, such as the mean, axial, and radial kurtoses. DKI is a diffusion-weighted MRI technique that allows the diffusional kurtosis to be estimated with clinical scanners using standard diffusion-weighted pulse sequences and relatively modest acquisition times. DKI is an extension of the widely used diffusion tensor imaging method, but requires the use of at least 3 b-values and 15 diffusion directions. This review discusses the underlying theory of DKI as well as practical considerations related to data acquisition and post-processing. It is argued that the diffusional kurtosis is sensitive to diffusional heterogeneity and suggested that DKI may be useful for investigating ischemic stroke and neuropathologies, such as Alzheimer's disease and schizophrenia.
Copyright © 2010 John Wiley & Sons, Ltd.
Figures


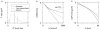
References
-
- Tanner JE, Stejskal EO. Restricted self-diffusion of protons in colloidal systems by the pulsed-gradient, spin-echo method. J Chem Phys. 1968;49:1768–1777.
-
- DeCarlo LT. On the meaning and use of kurtosis. Psychol Meth. 1997;2:292–307.
-
- Jensen JH, Helpern JA. Quantifying non-Gaussian water diffusion by means of pulsed-field-gradient MRI. Proceedings of the 11th Annual Meeting of ISMRM; Toronto, Canada. 2003. p. 2154.
-
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–1440. - PubMed
-
- Lu H, Jensen JH, Ramani A, Helpern JA. Three-dimensional characterization of non-Gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006;19:236–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

