Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics
- PMID: 20632964
- PMCID: PMC3066024
- DOI: 10.2174/187152710793361586
Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics
Abstract
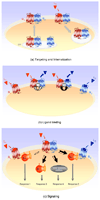
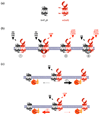
Because G protein-coupled receptors (GPCRs) are numerous, widely expressed and involved in major physiological responses, they represent a relevant therapeutic target for drug discovery, particularly regarding pharmacological treatments of neurological disorders. Among the biological phenomena regulating receptor function, GPCR heteromerization is an important emerging area of interest and investigation. There is increasing evidence showing that heteromerization contributes to the pharmacological heterogeneity of GPCRs by modulating receptor ontogeny, activation and recycling. Although in many cases the physiological relevance of receptor heteromerization has not been fully established, the unique pharmacological and functional properties of heteromers are likely to lead to new strategies in clinical medicine. This review describes the main GPCR heteromers and their implications for major neurological disorders such as Parkinson's disease, schizophrenia and addiction. A better understanding of molecular mechanisms underlying drug interactions related to the targeting of receptor heteromers could provide more specific and efficient therapeutic agents for the treatment of brain diseases.
Figures

References
-
- Milligan G, Bouvier M. Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J. 2005;272:2914–2925. - PubMed
-
- El-Asmar L, Springael JY, Ballet S, Andrieu EU, Vassart G, Parmentier M. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol. Pharmacol. 2005;67:460–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
