Hepatitis C: viral and host factors associated with non-response to pegylated interferon plus ribavirin
- PMID: 20633102
- PMCID: PMC3071938
- DOI: 10.1111/j.1478-3231.2010.02283.x
Hepatitis C: viral and host factors associated with non-response to pegylated interferon plus ribavirin
Abstract
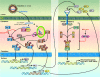
Treatment for chronic hepatitis C virus (HCV) infection has evolved considerably in the last years. The standard of care (SOC) for HCV infection consists in the combination of pegylated interferon (PEG-IFN) plus ribavirin. However, it only induces a sustained virological response (SVR) in half of genotype 1-infected patients. Several viral and host factors have been associated with non-response: steatosis, obesity, insulin resistance, age, male sex, ethnicity and genotypes. Many studies have demonstrated that in non-responders, some interferon-stimulated genes were upregulated before treatment. Those findings associated to clinical, biochemical and histological data may help detect responders before starting any treatment. This is a very important issue because the standard treatment is physically and economically demanding. The future of HCV treatment would probably consist in the addition of specifically targeted antiviral therapy for HCV such as protease and/or polymerase inhibitors to the SOC. In genotype 1 patients, very promising results have been reported when the protease inhibitor telaprevir or boceprevir is added to the SOC. It increases the SVR rates from approximately 50% (PEG-IFN plus ribavirin) to 70% (for patients treated with a combination of PEG-IFN plus ribavirin plus telaprevir). Different elements are associated with non-response: (i) viral factors, (ii) host factors and (iii) molecular mechanisms induced by HCV proteins to inhibit the IFN signalling pathway. The goal of this review is to present the mechanisms of non-response, to overcome it and to identify factors that can help to predict the response to anti-HCV therapy.
© 2010 John Wiley & Sons A/S.
Figures


References
-
- Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–46. - PubMed
-
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl. 1):74–81. - PubMed
-
- Marcellin P. Hepatitis B and hepatitis C in 2009. Liver Int. 2009;29(Suppl. 1):1–8. - PubMed
-
- Choo QL, Kuo G, Weiner AJ, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62. - PubMed
-
- Simmonds P, Bukh J, Combet C, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

