Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-alpha production
- PMID: 20633260
- PMCID: PMC2915995
- DOI: 10.1186/1471-2334-10-209
Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-alpha production
Abstract
Background: Nitric oxide (NO) produced in macrophages plays a pivotal role as a leishmanicidal agent. A previous study has demonstrated that 20% of the L. (V.) braziliensis isolated from initial cutaneous lesions of patients from the endemic area of Corte de Pedra, Bahia, Brazil, were NO resistant. Additionally, 5 to 11% of the patients did not respond to three or more antimony treatments" (refractory patients). The aim of this study is to investigate if there is an association between the resistance of L. (V.) braziliensis to NO and nonresponsiveness to antimony therapy and cytokine production.
Methods: We evaluated the in vitro toxicity of NO against the promastigotes stages of L. (V.) braziliensis isolated from responsive and refractory patients, and the infectivity of the amastigote forms of these isolates against human macrophages. The supernatants from Leishmania infected macrophage were used to measure TNF-alpha and IL-10 levels.
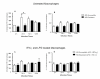
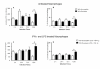
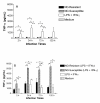
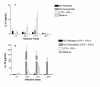
Results: Using NaNO2 (pH 5.0) as the NO source, L. (V.) braziliensis isolated from refractory patients were more NO resistant (IC50 = 5.8 +/- 4.8) than L. (V.) braziliensis isolated from responsive patients (IC50 = 2.0 +/- 1.4). Four isolates were selected to infect human macrophages: NO-susceptible and NO-resistant L. (V.) braziliensis isolated from responsive and refractory patients. NO-resistant L. (V.) braziliensis isolated from refractory patients infected more macrophages stimulated with LPS and IFN-gamma at 120 hours than NO-susceptible L. (V.) braziliensis isolated from refractory patients. Also, lower levels of TNF-alpha were detected in supernatants of macrophages infected with NO-resistant L. (V.) braziliensis as compared to macrophages infected with NO-susceptible L. (V.) braziliensis (p < 0.05 at 2, 24 and 120 hours), while no differences were detected in IL-10 levels.
Conclusion: These data suggest that NO resistance could be related to the nonresponsiveness to antimony therapy seen in American Tegumentary Leishmaniasis.
Figures

References
-
- Grimaldi G Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. - PubMed
-
- Carvalho LP, Passos ST, Jesus AR. Imunopatogênese da Leishmaniose Tegumentar. Gaz Med Bahia. 2005;1:57–65.
-
- Castes M, Trujillo D, Rojas ME, Fernandez CT, Araya L, Cabrera M, Blackwell J, Convit J. Serum levels of tumor necrosis factor in patients with American cutaneous leishmaniasis. Biol Res. 1993;26:233–238. - PubMed
-
- Costa JM, Marsden PD, Llanos-Cuentas EA, Netto EM, Carvalho EM, Barral A, Rosa AC, Cuba CC, Magalhaes AV, Barreto AC. Disseminated cutaneous leishmaniasis in a field clinic in Bahia Brazil: a report of eight cases. J Trop Med Hyg. 1986;89:319–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

