A Cajal body-independent pathway for telomerase trafficking in mice
- PMID: 20633556
- PMCID: PMC2945411
- DOI: 10.1016/j.yexcr.2010.07.001
A Cajal body-independent pathway for telomerase trafficking in mice
Abstract
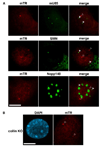
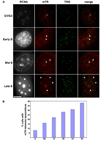
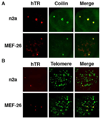
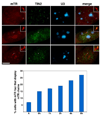
The intranuclear trafficking of human telomerase involves a dynamic interplay between multiple nuclear sites, most notably Cajal bodies and telomeres. Cajal bodies are proposed to serve as sites of telomerase maturation, storage, and assembly, as well as to function in the cell cycle-regulated delivery of telomerase to telomeres in human cells. Here, we find that telomerase RNA does not localize to Cajal bodies in mouse cells, and instead resides in separate nuclear foci throughout much of the cell cycle. However, as in humans, mouse telomerase RNA (mTR) localizes to subsets of telomeres specifically during S phase. The localization of mTR to telomeres in mouse cells does not require coilin-containing Cajal bodies, as mTR is found at telomeres at similar frequencies in cells from wild-type and coilin knockout mice. At the same time, we find that human TR localizes to Cajal bodies (as well as telomeres) in mouse cells, indicating that the distinct trafficking of mTR is attributable to an intrinsic property of the RNA (rather than a difference in the mouse cell environment such as the properties of mouse Cajal bodies). We also find that during S phase, mTR foci coalesce into short chains, with at least one of the conjoined mTR foci co-localizing with a telomere. These findings point to a novel, Cajal body-independent pathway for telomerase biogenesis and trafficking in mice.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. - PubMed
-
- Artandi SE. Telomeres, telomerase, and human disease. N Engl J Med. 2006;355:1195–1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

