Dissecting the functional specificities of two Hox proteins
- PMID: 20634319
- PMCID: PMC2904943
- DOI: 10.1101/gad.1936910
Dissecting the functional specificities of two Hox proteins
Abstract
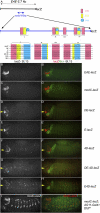
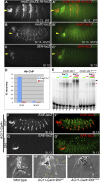
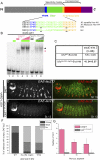
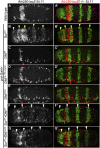
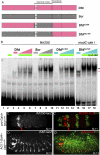
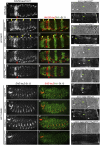
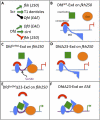
Hox proteins frequently select and regulate their specific target genes with the help of cofactors like Extradenticle (Exd) and Homothorax (Hth). For the Drosophila Hox protein Sex combs reduced (Scr), Exd has been shown to position a normally unstructured portion of Scr so that two basic amino acid side chains can insert into the minor groove of an Scr-specific DNA-binding site. Here we provide evidence that another Drosophila Hox protein, Deformed (Dfd), uses a very similar mechanism to achieve specificity in vivo, thus generalizing this mechanism. Furthermore, we show that subtle differences in the way Dfd and Scr recognize their specific binding sites, in conjunction with non-DNA-binding domains, influence whether the target gene is transcriptionally activated or repressed. These results suggest that the interaction between these DNA-binding proteins and the DNA-binding site determines the architecture of the Hox-cofactor-DNA ternary complex, which in turn determines whether the complex recruits coactivators or corepressors.
Figures
Similar articles
-
The control of trunk Hox specificity and activity by Extradenticle.Genes Dev. 1999 Jul 1;13(13):1704-16. doi: 10.1101/gad.13.13.1704. Genes Dev. 1999. PMID: 10398683 Free PMC article.
-
Regulation of Hox target genes by a DNA bound Homothorax/Hox/Extradenticle complex.Development. 1999 Nov;126(22):5137-48. doi: 10.1242/dev.126.22.5137. Development. 1999. PMID: 10529430
-
Hox repression of a target gene: extradenticle-independent, additive action through multiple monomer binding sites.Development. 2002 Jul;129(13):3115-26. doi: 10.1242/dev.129.13.3115. Development. 2002. PMID: 12070087
-
Cellular analysis of newly identified Hox downstream genes in Drosophila.Eur J Cell Biol. 2010 Feb-Mar;89(2-3):273-8. doi: 10.1016/j.ejcb.2009.11.012. Epub 2009 Dec 16. Eur J Cell Biol. 2010. PMID: 20018403 Review.
-
The specificity of homeotic gene function.Bioessays. 1995 Oct;17(10):855-63. doi: 10.1002/bies.950171007. Bioessays. 1995. PMID: 7487967 Review.
Cited by
-
The cis-regulatory logic underlying abdominal Hox-mediated repression versus activation of regulatory elements in Drosophila.Dev Biol. 2019 Jan 15;445(2):226-236. doi: 10.1016/j.ydbio.2018.11.006. Epub 2018 Nov 20. Dev Biol. 2019. PMID: 30468713 Free PMC article.
-
Competition for cofactor-dependent DNA binding underlies Hox phenotypic suppression.Genes Dev. 2011 Nov 15;25(22):2327-32. doi: 10.1101/gad.175539.111. Genes Dev. 2011. PMID: 22085961 Free PMC article.
-
Hox proteins display a common and ancestral ability to diversify their interaction mode with the PBC class cofactors.PLoS Biol. 2012;10(6):e1001351. doi: 10.1371/journal.pbio.1001351. Epub 2012 Jun 26. PLoS Biol. 2012. PMID: 22745600 Free PMC article.
-
The cis-regulatory code of Hox function in Drosophila.EMBO J. 2012 Aug 1;31(15):3323-33. doi: 10.1038/emboj.2012.179. Epub 2012 Jul 10. EMBO J. 2012. PMID: 22781127 Free PMC article.
-
Binding polymorphism in the DNA bound state of the Pdx1 homeodomain.PLoS Comput Biol. 2013;9(8):e1003160. doi: 10.1371/journal.pcbi.1003160. Epub 2013 Aug 8. PLoS Comput Biol. 2013. PMID: 23950697 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
