Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs
- PMID: 20634375
- PMCID: PMC3321833
- DOI: 10.1182/blood-2009-10-249177
Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs
Abstract
Secondary lymphoid organs provide a unique microenvironment for generation of immune responses. Using a cell type-specific conditional knockout approach, we have dissected contributions of tumor necrosis factor (TNF) produced by B cells (B-TNF) or T cells (T-TNF) to the genesis and homeostatic organization of secondary lymphoid organs. In spleen, lymph nodes and Peyer patches, the cellular source of TNF, and its molecular form (soluble versus membrane-bound) appeared distinct. In spleen, in addition to major B-TNF signal, a complementary T-TNF signal contributed to the microstructure. In contrast, B-TNF predominantly controlled the development of follicular dendritic cells and B-cell follicles in Peyer patches. In lymph nodes, cooperation between TNF expressed by B and T cells was necessary for the maintenance of microarchitecture and for generation of an efficient humoral immune response. Unexpectedly, soluble but not membrane TNF expressed by B cells was essential for the organization of the secondary lymphoid organs. Thus, the maintenance of each type of secondary lymphoid organ is orchestrated by distinct contributions of membrane-bound and soluble TNF produced by B and T lymphocytes.
Figures
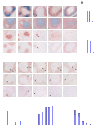
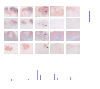


References
-
- Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. - PubMed
-
- Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3(4):292–303. - PubMed
-
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315(5811):528–531. - PubMed
-
- Tumanov AV, Grivennikov SI, Shakhov AN, et al. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol Rev. 2003;195:106–116. - PubMed
-
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

