The structural plasticity of SCA7 domains defines their differential nucleosome-binding properties
- PMID: 20634802
- PMCID: PMC2920448
- DOI: 10.1038/embor.2010.98
The structural plasticity of SCA7 domains defines their differential nucleosome-binding properties
Abstract
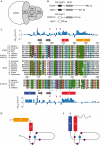
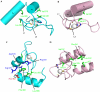
SAGA (Spt-Ada-Gcn5 acetyltransferase), a coactivator complex involved in chromatin remodelling, harbours both histone acetylation and deubiquitination activities. ATXN7/Sgf73 and ATXN7L3, two subunits of the SAGA deubiquitination module, contain an SCA7 domain characterized by an atypical zinc-finger. We show that the yeast Sgf73-SCA7 domain is not required to recruit Sgf73 into SAGA. Instead, it binds to nucleosomes, a property that is conserved in the human ATXN7-SCA7 domain but is lost in the ATXN7L3 domain. The solution structures of the SCA7 domain of both ATXN7 and ATXN7L3 reveal a new, common zinc-finger motif at the heart of two distinct folds, providing a molecular basis for the observed functional differences.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Bonnet J, Romier C, Tora L, Devys D (2008) Zinc-finger UBPs: regulators of deubiquitylation. Trends Biochem Sci 33: 369–375 - PubMed
-
- Cornilescu G, Delaglio F, Bax A (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR 13: 289–302 - PubMed
-
- Daniel JA, Torok MS, Sun ZW, Schieltz D, Allis CD, Yates JR III, Grant PA (2004) Deubiquitination of histone H2B by a yeast acetyltransferase complex regulates transcription. J Biol Chem 279: 1867–1871 - PubMed
-
- Helmlinger D et al. (2004) Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes. Hum Mol Genet 13: 1257–1265 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

