Multimodal functional neuroimaging: integrating functional MRI and EEG/MEG
- PMID: 20634915
- PMCID: PMC2903760
- DOI: 10.1109/RBME.2008.2008233
Multimodal functional neuroimaging: integrating functional MRI and EEG/MEG
Abstract
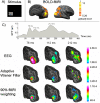
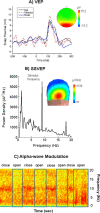
Noninvasive functional neuroimaging, as an important tool for basic neuroscience research and clinical diagnosis, continues to face the need of improving the spatial and temporal resolution. While existing neuroimaging modalities might approach their limits in imaging capability mostly due to fundamental as well as technical reasons, it becomes increasingly attractive to integrate multiple complementary modalities in an attempt to significantly enhance the spatiotemporal resolution that cannot be achieved by any modality individually. Electrophysiological and hemodynamic/metabolic signals reflect distinct but closely coupled aspects of the underlying neural activity. Combining fMRI and EEG/MEG data allows us to study brain function from different perspectives. In this review, we start with an overview of the physiological origins of EEG/MEG and fMRI, as well as their fundamental biophysics and imaging principles, we proceed with a review of the major advances in the understanding and modeling of neurovascular coupling and in the methodologies for the fMRI-EEG/MEG simultaneous recording. Finally, we summarize important remaining issues and perspectives concerning multimodal functional neuroimaging, including brain connectivity imaging.
Keywords: EEG; MEG; Multimodal neuroimaging; fMRI; human brain mapping; neurovascular coupling.
Figures






References
-
- Berger H. Ü ber das Elektroenkephalogram des Menschen. Arch. f. Psychiat. 1929;87:527–70.
-
- Cohen D. Magnetoencephalography: evidence of magnetic fields produced by alpha-rhythm currents. Science. 1968 Aug 23;161:784–786. - PubMed
-
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn. Reson. Med. 1992 Jun;25:390–397. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

