Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse
- PMID: 20634957
- PMCID: PMC2902518
- DOI: 10.1371/journal.pone.0011524
Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse
Abstract
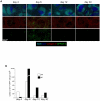
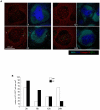
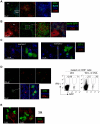
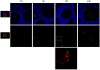
Memory CD8(+) T cells represent an important effector arm of the immune response in maintaining long-lived protective immunity against viruses and some intracellular bacteria such as Listeria monocytogenes (L.m). Memory CD8(+) T cells are endowed with enhanced antimicrobial effector functions that perfectly tail them to rapidly eradicate invading pathogens. It is largely accepted that these functions are sufficient to explain how memory CD8(+) T cells can mediate rapid protection. However, it is important to point out that such improved functional features would be useless if memory cells were unable to rapidly find the pathogen loaded/infected cells within the infected organ. Growing evidences suggest that the anatomy of secondary lymphoid organs (SLOs) fosters the cellular interactions required to initiate naive adaptive immune responses. However, very little is known on how the SLOs structures regulate memory immune responses. Using Listeria monocytogenes (L.m) as a murine infection model and imaging techniques, we have investigated if and how the architecture of the spleen plays a role in the reactivation of memory CD8(+) T cells and the subsequent control of L.m growth. We observed that in the mouse, memory CD8(+) T cells start to control L.m burden 6 hours after the challenge infection. At this very early time point, L.m-specific and non-specific memory CD8(+) T cells localize in the splenic red pulp and form clusters around L.m infected cells while naïve CD8(+) T cells remain in the white pulp. Within these clusters that only last few hours, memory CD8(+) T produce inflammatory cytokines such as IFN-gamma and CCL3 nearby infected myeloid cells known to be crucial for L.m killing. Altogether, we describe how memory CD8(+) T cells trafficking properties and the splenic micro-anatomy conjugate to create a spatio-temporal window during which memory CD8(+) T cells provide a local response by secreting effector molecules around infected cells.
Conflict of interest statement
Figures







References
-
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. - PubMed
-
- Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. - PubMed
-
- Young AJ. The physiology of lymphocyte migration through the single lymph node in vivo. Semin Immunol. 1999;11:73–83. - PubMed
-
- Bajenoff M, Egen JG, Qi H, Huang AY, Castellino F, et al. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28:346–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

