Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes
- PMID: 20635156
- PMCID: PMC2949076
- DOI: 10.1007/s10875-010-9441-2
Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes
Abstract
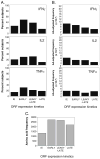
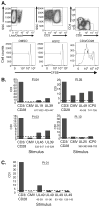
CD8(+) T cells are known to be important in clearing herpes simplex virus (HSV) infections. However, investigating the specific antiviral mechanisms employed by HSV-2-specific T cell populations is limited by a lack of reagents such as CD8(+) T cell epitopes and specific tetramers. Using a combination of intracellular cytokine staining flow cytometry and ELISpot methods, we functionally characterized peripheral HSV-2-specific CD8(+) T cells from peripheral blood mononuclear cell (PBMC) that recognize 14 selected HSV-2 open-reading frames (ORFs) from 55 HSV-2 seropositive persons; within these ORFs, we subsequently identified more than 20 unique CD8(+) T cell epitopes. CD8(+) T cells to HSV-2 exhibited significant heterogeneity in their functional characteristics, proliferation, production of inflammatory cytokines, and potential to degranulate ex vivo. The diversity in T cell response in these ex vivo assessments offers the potential of defining immune correlates of HSV-2 reactivation in humans.
Figures






References
-
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. - PubMed
-
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

