Reduced electromotility of outer hair cells associated with connexin-related forms of deafness: an in silico study of a cochlear network mechanism
- PMID: 20635191
- PMCID: PMC2975888
- DOI: 10.1007/s10162-010-0226-3
Reduced electromotility of outer hair cells associated with connexin-related forms of deafness: an in silico study of a cochlear network mechanism
Abstract
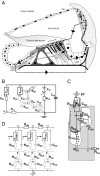
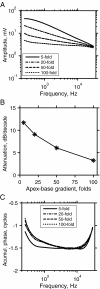
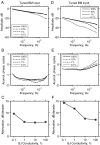
Mutations in the GJB2 gene encoding for the connexin 26 (Cx26) protein are the most common source of nonsyndromic forms of deafness. Cx26 is a building block of gap junctions (GJs) which establish electrical connectivity in distinct cochlear compartments by allowing intercellular ionic (and metabolic) exchange. Animal models of the Cx26 deficiency in the organ of Corti seem to suggest that the hearing loss and the degeneration of outer hair cells (OHCs) and inner hair cells is due to failed K(+) and metabolite homeostasis. However, OHCs can develop normally in some mutants, suggesting that the hair cells death is not the universal mechanism. In search for alternatives, we have developed an in silico large scale three-dimensional model of electrical current flow in the cochlea in the small signal, linearised, regime. The effect of mutations was analysed by varying the magnitude of resistive components representing the GJ network in the organ of Corti. The simulations indeed show that reduced GJ conductivity increases the attenuation of the OHC transmembrane potential at frequencies above 5 kHz from 6.1 dB/decade in the wild-type to 14.2 dB/decade. As a consequence of increased GJ electrical filtering, the OHC transmembrane potential is reduced by up to 35 dB at frequencies >10 kHz. OHC electromotility, driven by this potential, is crucial for sound amplification, cochlear sensitivity and frequency selectivity. Therefore, we conclude that reduced OHC electromotility may represent an additional mechanism underlying deafness in the presence of Cx26 mutations and may explain lowered OHC functionality in particular reported Cx26 mutants.
Figures




Similar articles
-
A Gap-Junction Mutation Reveals That Outer Hair Cell Extracellular Receptor Potentials Drive High-Frequency Cochlear Amplification.J Neurosci. 2022 Oct 19;42(42):7875-7884. doi: 10.1523/JNEUROSCI.2241-21.2022. Epub 2022 Sep 9. J Neurosci. 2022. PMID: 36261265 Free PMC article.
-
A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: Reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall.Neurobiol Dis. 2017 Dec;108:195-203. doi: 10.1016/j.nbd.2017.08.002. Epub 2017 Aug 17. Neurobiol Dis. 2017. PMID: 28823936 Free PMC article.
-
Cochlear outer hair cells in a dominant-negative connexin26 mutant mouse preserve non-linear capacitance in spite of impaired distortion product otoacoustic emission.Neuroscience. 2009 Dec 15;164(3):1312-9. doi: 10.1016/j.neuroscience.2009.08.043. Epub 2009 Aug 25. Neuroscience. 2009. PMID: 19712724
-
Connexins, hearing and deafness: clinical aspects of mutations in the connexin 26 gene.Brain Res Brain Res Rev. 2000 Apr;32(1):159-62. doi: 10.1016/s0165-0173(99)00075-2. Brain Res Brain Res Rev. 2000. PMID: 10928803 Review.
-
Prestin and electromotility may serve multiple roles in cochlear outer hair cells.Hear Res. 2022 Sep 15;423:108428. doi: 10.1016/j.heares.2021.108428. Epub 2021 Dec 26. Hear Res. 2022. PMID: 34987016 Review.
Cited by
-
Molecular Evaluation of Joubert Syndrome and Hearing Impairment in a Patient with Ataxic Cerebral Palsy.Glob Med Genet. 2023 Jul 17;10(3):190-193. doi: 10.1055/s-0043-1771184. eCollection 2023 Sep. Glob Med Genet. 2023. PMID: 37501760 Free PMC article.
-
Age-dependent gene expression in the inner ear of big brown bats (Eptesicus fuscus).PLoS One. 2017 Oct 26;12(10):e0186667. doi: 10.1371/journal.pone.0186667. eCollection 2017. PLoS One. 2017. PMID: 29073148 Free PMC article.
-
Purinergic Signaling and Cochlear Injury-Targeting the Immune System?Int J Mol Sci. 2019 Jun 18;20(12):2979. doi: 10.3390/ijms20122979. Int J Mol Sci. 2019. PMID: 31216722 Free PMC article. Review.
-
Active cochlear amplification is dependent on supporting cell gap junctions.Nat Commun. 2013;4:1786. doi: 10.1038/ncomms2806. Nat Commun. 2013. PMID: 23653198 Free PMC article.
-
Inner Ear Connexin Channels: Roles in Development and Maintenance of Cochlear Function.Cold Spring Harb Perspect Med. 2019 Jul 1;9(7):a033233. doi: 10.1101/cshperspect.a033233. Cold Spring Harb Perspect Med. 2019. PMID: 30181354 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

