Hypoxia and hypoglycaemia in Ewing's sarcoma and osteosarcoma: regulation and phenotypic effects of Hypoxia-Inducible Factor
- PMID: 20637078
- PMCID: PMC2918574
- DOI: 10.1186/1471-2407-10-372
Hypoxia and hypoglycaemia in Ewing's sarcoma and osteosarcoma: regulation and phenotypic effects of Hypoxia-Inducible Factor
Abstract
Background: Hypoxia regulates gene expression via the transcription factor HIF (Hypoxia-Inducible Factor). Little is known regarding HIF expression and function in primary bone sarcomas. We describe HIF expression and phenotypic effects of hypoxia, hypoglycaemia and HIF in Ewing's sarcoma and osteosarcoma.
Methods: HIF-1alpha and HIF-2alpha immunohistochemistry was performed on a Ewing's tumour tissue array. Ewing's sarcoma and osteosarcoma cell lines were assessed for HIF pathway induction by Western blot, luciferase assay and ELISA. Effects of hypoxia, hypoglycaemia and isoform-specific HIF siRNA were assessed on proliferation, apoptosis and migration.
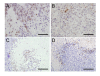
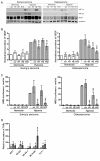
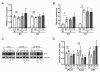
Results: 17/56 Ewing's tumours were HIF-1alpha-positive, 15 HIF-2alpha-positive and 10 positive for HIF-1alpha and HIF-2alpha. Expression of HIF-1alpha and cleaved caspase 3 localised to necrotic areas. Hypoxia induced HIF-1alpha and HIF-2alpha in Ewing's and osteosarcoma cell lines while hypoglycaemia specifically induced HIF-2alpha in Ewing's. Downstream transcription was HIF-1alpha-dependent in Ewing's sarcoma, but regulated by both isoforms in osteosarcoma. In both cell types hypoglycaemia reduced cellular proliferation by >or= 45%, hypoxia increased apoptosis and HIF siRNA modulated hypoxic proliferation and migration.
Conclusions: Co-localisation of HIF-1alpha and necrosis in Ewing's sarcoma suggests a role for hypoxia and/or hypoglycaemia in in vivo induction of HIF. In vitro data implicates hypoxia as the primary HIF stimulus in both Ewing's and osteosarcoma, driving effects on proliferation and apoptosis. These results provide a foundation from which to advance understanding of HIF function in the pathobiology of primary bone sarcomas.
Figures

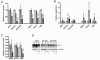
Similar articles
-
EWS/Fli-1 chimeric fusion gene upregulates vascular endothelial growth factor-A.Int J Cancer. 2010 Jun 15;126(12):2790-8. doi: 10.1002/ijc.24781. Int J Cancer. 2010. PMID: 19642105
-
Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing's sarcoma cells in vitro.Cancer Res. 2010 May 15;70(10):4015-23. doi: 10.1158/0008-5472.CAN-09-4333. Epub 2010 May 4. Cancer Res. 2010. PMID: 20442286 Free PMC article.
-
Multiple receptor tyrosine kinases regulate HIF-1alpha and HIF-2alpha in normoxia and hypoxia in neuroblastoma: implications for antiangiogenic mechanisms of multikinase inhibitors.Oncogene. 2010 May 20;29(20):2938-49. doi: 10.1038/onc.2010.60. Epub 2010 Mar 8. Oncogene. 2010. PMID: 20208561
-
Impact of ABC Transporters in Osteosarcoma and Ewing's Sarcoma: Which Are Involved in Chemoresistance and Which Are Not?Cells. 2021 Sep 17;10(9):2461. doi: 10.3390/cells10092461. Cells. 2021. PMID: 34572110 Free PMC article. Review.
-
Effects of hypoxia-inducible factor-1α and hypoxia-inducible factor-2α overexpression on hepatocellular carcinoma survival: A systematic review with meta-analysis.J Gastroenterol Hepatol. 2021 Jun;36(6):1487-1496. doi: 10.1111/jgh.15395. Epub 2021 Jan 26. J Gastroenterol Hepatol. 2021. PMID: 33393670
Cited by
-
The acetate/ACSS2 switch regulates HIF-2 stress signaling in the tumor cell microenvironment.PLoS One. 2015 Feb 17;10(2):e0116515. doi: 10.1371/journal.pone.0116515. eCollection 2015. PLoS One. 2015. PMID: 25689462 Free PMC article.
-
Angiopoietin-like 4 promotes osteosarcoma cell proliferation and migration and stimulates osteoclastogenesis.BMC Cancer. 2018 May 8;18(1):536. doi: 10.1186/s12885-018-4468-5. BMC Cancer. 2018. PMID: 29739381 Free PMC article.
-
Metabolic modulation of Ewing sarcoma cells inhibits tumor growth and stem cell properties.Oncotarget. 2017 Aug 24;8(44):77292-77308. doi: 10.18632/oncotarget.20467. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100387 Free PMC article.
-
Sphingosine Kinase-1 Is Overexpressed and Correlates with Hypoxia in Osteosarcoma: Relationship with Clinicopathological Parameters.Cancers (Basel). 2022 Jan 19;14(3):499. doi: 10.3390/cancers14030499. Cancers (Basel). 2022. PMID: 35158767 Free PMC article.
-
In Vivo Model for Testing Effect of Hypoxia on Tumor Metastasis.J Vis Exp. 2016 Dec 9;(118):54532. doi: 10.3791/54532. J Vis Exp. 2016. PMID: 28060251 Free PMC article.
References
-
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/S0092-8674(01)00507-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

