An olfactory subsystem that detects carbon disulfide and mediates food-related social learning
- PMID: 20637621
- PMCID: PMC2929674
- DOI: 10.1016/j.cub.2010.06.021
An olfactory subsystem that detects carbon disulfide and mediates food-related social learning
Abstract
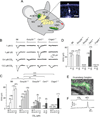
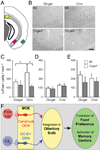
Olfactory signals influence social interactions in a variety of species. In mammals, pheromones and other social cues can promote mating or aggression behaviors; can communicate information about social hierarchies, genetic identity and health status; and can contribute to associative learning. However, the molecular, cellular, and neural mechanisms underlying many olfactory-mediated social interactions remain poorly understood. Here, we report that a specialized olfactory subsystem that includes olfactory sensory neurons (OSNs) expressing the receptor guanylyl cyclase GC-D, the cyclic nucleotide-gated channel subunit CNGA3, and the carbonic anhydrase isoform CAII (GC-D(+) OSNs) is required for the acquisition of socially transmitted food preferences (STFPs) in mice. Using electrophysiological recordings from gene-targeted mice, we show that GC-D(+) OSNs are highly sensitive to the volatile semiochemical carbon disulfide (CS(2)), a component of rodent breath and a known social signal mediating the acquisition of STFPs. Olfactory responses to CS(2) are drastically reduced in mice lacking GC-D, CNGA3, or CAII. Disruption of this sensory transduction cascade also results in a failure to acquire STFPs from either live or surrogate demonstrator mice or to exhibit hippocampal correlates of STFP retrieval. Our findings indicate that GC-D(+) OSNs detect chemosignals that facilitate food-related social interactions.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. - PubMed
-
- Cheal ML, Sprott RL. Social olfaction: a review of the role of olfaction in a variety of animal behaviors. Psychol Rep. 1971;29:195–243. - PubMed
-
- Restrepo D, Lin W, Salcedo E, Yamazaki K, Beauchamp G. Odortypes and MHC peptides: Complementary chemosignals of MHC haplotype? Trends Neurosci. 2006;29:604–609. - PubMed
-
- Kavaliers M, Choleris E, Pfaff DW. Genes, odours and the recognition of parasitized individuals by rodents. Trends Parasitol. 2005;21:423–429. - PubMed
-
- Leon M. The neurobiology of filial learning. Annu Rev Psychol. 1992;43:377–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

