Functional skeletal muscle formation with a biologic scaffold
- PMID: 20638716
- PMCID: PMC2922042
- DOI: 10.1016/j.biomaterials.2010.06.039
Functional skeletal muscle formation with a biologic scaffold
Abstract
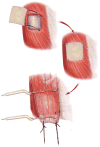
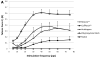
Biologic scaffolds composed of extracellular matrix (ECM) have been used to reinforce or replace damaged or missing musculotendinous tissues in both preclinical studies and in human clinical applications. However, most studies have focused upon morphologic endpoints and few studies have assessed the in-situ functionality of newly formed tissue; especially new skeletal muscle tissue. The objective of the present study was to determine both the in-situ tetanic contractile response and histomorphologic characteristics of skeletal muscle tissue reconstructed using one of four test articles in a rodent abdominal wall model: 1) porcine small intestinal submucosa (SIS)-ECM; 2) carbodiimide-crosslinked porcine SIS-ECM; 3) autologous tissue; or 4) polypropylene mesh. Six months after surgery, the remodeled SIS-ECM showed almost complete replacement by islands and sheets of skeletal muscle, which generated a similar maximal contractile force to native tissue but with greater resistance to fatigue. The autologous tissue graft was replaced by a mixture of collagenous connective tissue, adipose tissue with fewer islands of skeletal muscle compared to SIS-ECM and a similar fatigue resistance to native muscle. Carbodiimide-crosslinked SIS-ECM and polypropylene mesh were characterized by a chronic inflammatory response and produced little or no measurable tetanic force. The findings of this study show that non-crosslinked xenogeneic SIS scaffolds and autologous tissue are associated with the restoration of functional skeletal muscle with histomorphologic characteristics that resemble native muscle.
2010 Elsevier Ltd. All rights reserved.
Figures









References
-
- Akhyari P, Kamiya H, Haverich A, Karck M, Lichtenberg A. Myocardial tissue engineering: the extracellular matrix. Eur J Cardiothorac Surg. 2008;34:229–41. - PubMed
-
- Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, et al. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15 1:S29–40. - PubMed
-
- Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I144–9. - PubMed
-
- Nieponice A, Gilbert TW, Badylak SF. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg. 2006;82:2050–8. - PubMed
-
- Nieponice A, McGrath K, Qureshi I, Beckman EJ, Luketich JD, Gilbert TW, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2008;69:289–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

