Developmental origin of vaginal epithelium
- PMID: 20638775
- PMCID: PMC2943051
- DOI: 10.1016/j.diff.2010.06.007
Developmental origin of vaginal epithelium
Abstract
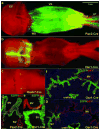
The developmental origin of vaginal epithelium has been controversial for nearly a century, with speculation that vaginal epithelium originates from the Müllerian duct, Wolffian duct, and/or urogenital sinus. None of these possibilities have been definitively proven or disproven by direct scientific data. To define precisely the origin of vaginal epithelium, epithelial cells of the Müllerian duct, Wolffian duct, or urogenital sinus were fluorescently labeled in mouse embryos by crossing tdTomato-EGFP dual-reporter transgenic mice with transgenic mouse lines that express Cre-recombinase in each type of epithelium. In embryos and newborn mice, the vagina consisted of fused Müllerian ducts plus the sinus vagina of urogenital sinus origin. However, the proportion of the sinus vagina was significantly reduced as the Müllerian vagina grew caudally. By postpartum day 7, the Müllerian vagina extended to the caudal end of the body, whereas the sinus vagina remained only at the junction between the vagina and perineal skin. As the vagina opened in puberty, urogenital sinus epithelium was detected only in the vulva, but not in the vagina. Additionally, from embryo to adult stages, residual Wolffian duct epithelium was present in the dorsolateral stromal wall of the vagina, but not within vaginal or vulvar epithelium. In conclusion, adult mouse vaginal epithelium is derived solely from Müllerian duct epithelium.
Copyright © 2010 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures



Comment in
-
Derivation of vaginal epithelium finally resolved: broader implications regarding mechanism and pathogenic considerations.Differentiation. 2010 Sep-Oct;80(2-3):81. doi: 10.1016/j.diff.2010.07.002. Epub 2010 Jul 27. Differentiation. 2010. PMID: 20667647 No abstract available.
References
-
- Arey LB. Developmental anatomy: a textbook and laboratory manual of embryology. W.B. Saunders; Philadelphia: 1954.
-
- Boutin E, Sanderson R, Bernfield M, Cunha GR. Expression of syndecan, a cell surface proteoglycan, correlates with induced changes in cellular organization. The Journal of cell biology. 1989;107:605a.
-
- Boutin EL, Sanderson RD, Bernfield M, Cunha GR. Epithelial-mesenchymal interactions in uterus and vagina alter the expression of the cell surface proteoglycan, syndecan. Developmental biology. 1991;148:63–74. - PubMed
-
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Developmental cell. 2004;6:7–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

