Directed 3D cell alignment and elongation in microengineered hydrogels
- PMID: 20638973
- PMCID: PMC2908986
- DOI: 10.1016/j.biomaterials.2010.05.056
Directed 3D cell alignment and elongation in microengineered hydrogels
Abstract
Organized cellular alignment is critical to controlling tissue microarchitecture and biological function. Although a multitude of techniques have been described to control cellular alignment in 2D, recapitulating the cellular alignment of highly organized native tissues in 3D engineered tissues remains a challenge. While cellular alignment in engineered tissues can be induced through the use of external physical stimuli, there are few simple techniques for microscale control of cell behavior that are largely cell-driven. In this study we present a simple and direct method to control the alignment and elongation of fibroblasts, myoblasts, endothelial cells and cardiac stem cells encapsulated in microengineered 3D gelatin methacrylate (GelMA) hydrogels, demonstrating that cells with the intrinsic potential to form aligned tissues in vivo will self-organize into functional tissues in vitro if confined in the appropriate 3D microarchitecture. The presented system may be used as an in vitro model for investigating cell and tissue morphogenesis in 3D, as well as for creating tissue constructs with microscale control of 3D cellular alignment and elongation, that could have great potential for the engineering of functional tissues with aligned cells and anisotropic function.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


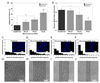
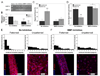



References
-
- Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: Molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280(1):H168–H178. - PubMed
-
- Wigmore PM, Dunglison GF. The generation of fiber diversity during myogenesis. Int J Dev Biol. 1998;42(2):117–125. - PubMed
-
- Chiu JJ, Chen LJ, Chen CN, Lee PL, Lee CI. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J Biomech. 2004;37(4):531–539. - PubMed
-
- Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004:6131–6156. - PubMed
-
- Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am. 2009;300(5):64–71. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

