Refining the definition of hypereosinophilic syndrome
- PMID: 20639008
- PMCID: PMC3400024
- DOI: 10.1016/j.jaci.2010.03.042
Refining the definition of hypereosinophilic syndrome
Abstract
Because of advances in our understanding of the hypereosinophilic syndrome (HES) and the availability of novel therapeutic agents, the original criteria defining these disorders are becoming increasingly problematic. Here, we discuss shortcomings with the current definition of HES and recent developments in the classification of these disorders. Despite significant progress in our understanding of the pathogenesis of some forms of HES, the current state of knowledge is still insufficient to formulate a new comprehensive etiologic definition of HESs. Nevertheless, we suggest a new working definition that overcomes some of the most obvious limitations with the original definition.
Conflict of interest statement
Disclosure of potential conflict of interest: H.-U. Simon has received research support from GlaxoSmithKline, AstraZeneca, Roche, CSL Behring, and Nycomed, and has provided legal consultation or expert witness testimony for Pfizer on the topic of general pharmacology. M. E. Rothenberg has consulted for and given talks for Merck; has consulted for Centocor, Ception Therapeutics, Nycomed, Array BioPharma, Biocrystal Pharmaceuticals, and Endo Pharmaceuticals; has received research support from the National Institutes of Health, the Food Allergy and Anaphylaxis Network, and the Dana Foundation; is on the medical advisory board of the American Partnership for Eosinophilic Diseases; and is on the executive council of the International Eosinophil Society. B. S. Bochner has consulted for Sanofi-Aventis and GlaxoSmithKline and has received research support from Sanofi-Aventis. P. F. Weller has received research support from Merck and has provided legal consultation services or expert witness testimony on the topic of eosinophilic diseases. A. J. Wardlaw is on advisory boards for GlaxoSmithKline and has received research support from GlaxoSmithKline, Pfizer, and AstraZeneca. M. E. Wechsler has consulted for and given talks for Genentech, Merck, and Novartis; has consulted for MedImmune, Medicinova, and GlaxoSmithKline; is on the data safety monitoring board for MAP Pharmaceuticals; and has received research support from the National Institutes of Health and GlaxoSmithKline. L. J. Rosenwasser has received research support from Novartis and Genentech and has consulted for Alcon, Sanofi-Aventis, GlaxoSmithKline, and AstraZeneca. F. Roufosse has consulted for GlaxoSmithKline. G. J. Gleich has provided legal consultation or expert witness testimony on the topic of heparin contamination and is on the board of directors for the American Partnership for Eosinophilic Disorders. A. D. Klion declares that she has no relevant conflicts of interest to disclose.
Figures
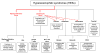
References
-
- Chusid MJ, Dale CD, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975;54:1–27. - PubMed
-
- Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–14. - PubMed
-
- Klion AD, Bochner BS, Gleich GJ, Nutman TB, Rothenberg ME, Simon HU, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117:1292–302. - PubMed
-
- Bain B, Pierre R, Imbert M, Vardiman JW, Brunning RD, Fladrin G. Chronic eosinophilic leukaemia and the hypereosinophilic syndrome. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization classification of tumours: pathology and genetics of tumours of hematopoietic and lymphoid tissue. Lyon: IARC Press; 2001. pp. 29–31.
-
- Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophilic disorders. Blood Rev. 2009;23:157–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

